Alekseeva N.S., Antypenko L.M.
Zaporizhzhya State Medical University, Ukraine
The
ethyl (5-R-([1,2,4]triazolo[1,5-c]quinazolin-2-yl)acetates
anticancer activity mechanism prediction
via docking studies
to thymidine
phosphorylase
The
thymidine phosphorylase (TP) also known as platelet derived endothelial cell
growth factor (PD-ECGF) is identified as a potential therapeutic target for
cancer therapy, because it plays a crucial role in cancer angiogenesis via the release of its metabolic
product, 2-deoxy-D-ribose, that stimulates the secretion and/or expression of
many angiogenic factors, including MMP-9, MMP-2, VEGF, IL-8 as well as
migration of endothelial cells and rapid formation of long-lasting functional
neo-vessels, leading to increased metastasis and tumour growth [1,2].
Considering
the presence of anticancer activity of the [1,2,4]triazolo[1,5-c]quinazoline derivatives [3], the prediction
of the anti-TP activity by docking studies will detect their potential mechanism
of action.
The
virtual library of ethyl (5-R-([1,2,4]triazolo[1,5-c]quinazolin-2-yl)acetates on the basis of [1,2,4]triazolo[1,5-c]quinazoline skeleton was created in silico with introduction of the
residues of thioalkyls, acids, esters, amides and amines (Pic.).
Investigation
was conducted by flexible molecular docking, as an approach of finding
molecules with affinity to a specific biological target using the software
package OpenEye, including related utilities: Fred Receptor2.2.5, Vida4.1.1,
Flipper, Babel3, Omega2.4.3 and Fred2.2.5 [4,5]. The crystal structure of the
enzyme TP (2WK6.pdb) was obtained from
the protein data bank [6]. The 1H-pyrrolo[2,3-d]pyrimidine-2,4-(3H,7H)-dione was used as
the reference [7].
The
methodology of research consisted of the following steps:
Ø generation of R-, S- and cis-,
trans-isomers of ligands (the studied
compounds and relevant drugs, program Flipper), which allowed the production
isomer’s range of studied compounds;
Ø generation of 3D-structure of the obtained isomeric forms - molecular
modelling (Hyper Chem 7.5) using the method of molecular mechanics (MM +) and
semiempirical quantum mechanical method with Polak-Ribiere algorithm (PM3);
Ø generation of conformations of ligands (Omega2.4.3). The number of
conformations obtained wasn’t significant due to the further selection by
program Fred2.2.5 most optimal conformer;
Ø carrying out molecular docking (Fred2.2.5).
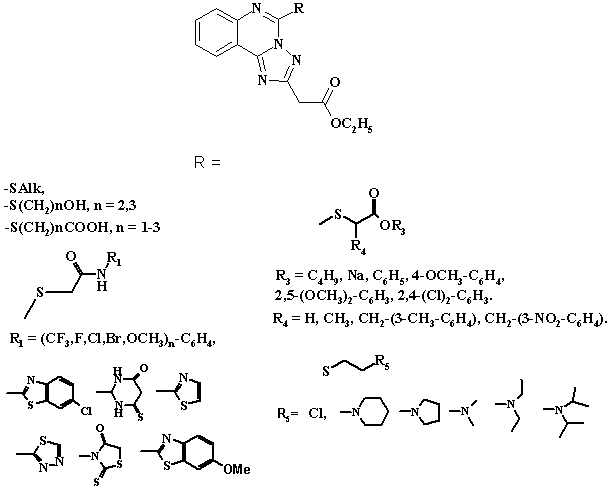
Pic.
Investigated ethyl (5-R-([1,2,4]triazolo[1,5-c]quinazolin-2-yl)acetates.
A
number of scoring functions (Shapegauss, PLP, Chemgauss2, Chemgauss3,
Chemscore, OEChemscore, Screenscore, CGO, CGT, Zapbind, Consensus Score) was
obtained as a result of studies, values of which assess specific
characteristics of the ligand-protein complex, indicating the possibility of
their matching.
The
analysis of docking interactions between TP and investigated compounds has
indicated that there is no possibility of their inlining into the
ligand-protein complex.
Hence,
in silico docking studies of the (5-R-([1,2,4]triazolo[1,5-c]quinazolin-2-yl)acetates
into the thymidine phosphorylase binding site showed
the absence of such activity of the designed structures.
Literature:
1.
Thymidine phosphorylase in cancer cells stimulates human endothelial cell
migration and invasion by the secretion of angiogenic factors. I.V. Bijnsdorp,
F. Capriotti, F.A.E. Kruyt, N. Losekoot, M. Fukushima, A.W. Griffioen,
V.L. Thijssen, G.J. Peters Br. J. Cancer
104 (2011) 1185–1192.
2.
J. Folkman. What is the role of thymidine phosphorylase in tumor angiogenesis? J. Natl.
Cancer Inst. 88 (1996) 1091–1092.
3. Synthesis
and anticancer activity of 2-àlkyl(alkaryl-,aryl-,heteryl-)-[1,2,4]triazolo[1,5-c]quinazolines. S.I. Kovalenko, L.M.
Antypenko, A.K. Bilyi, S.V. Kholodnyak, O.V. Karpenko, O.M. Antypenko, N.S.
Mykhaylova, T.I. Los’, O. S. Kîlîmîåts’. Sci.
Pharm., 81 (2013) 359-391.
4.
Virtual Screening in Drug Discovery, (Eds.: J. Alvarez, B. Shoichet), CRC
Press, Taylor & Francis, Boca Raton, FL, 2005, 451.
5.
Protein Data Bank, pdb., [http://www.pdb.org].
6.
Fred Receptor2.2.5, Vida4.1.1, Flipper, Babel3, Omega2.4.3 and Fred2.2.5:
OpenEye Sci. Soft. Inc. [http://www.eyesopen.com], Santa Fe, NM, USA; 2011.
7.
Synthesis, anti-thymidine phosphorylase activity and molecular docking of
5-thioxo-[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-ones
H. Bera, M.H. Lee, L.Sun, A.V. Dolzhenko, W.K. Chui Bioorg. Chem. 50 (2013) 34–40.