technical / Processing of materials in mechanical engineering
Nurmukhanova A.Z. Candidate of technical sciences, Mukhtarova
M.N. Candidate of physical and
mathematical sciences, Nurseytova A.K.,
Konakbaev B.O.,
Zulbukharova E.M., Ermaganbetova S. D.
Kazakh National University named after Al-Farabi, City
of Almaty
Republic of Kazakhstan
ANALYSIS OF PHYSICAL AND MECHANICAL
PROPERTIES OF MATERIALS NONMETALLIC
This paper analyzes the determination,
dignity, non-metallic materials basis and classification of polymers. Also
provides a method for producing polymers, which are divided into the
polymerization and polycondensation. Describes the process of polymerization of
the chemical compound and a large number of molecules of the monomer in a
larger polymer molecule without changing the elemental composition of the
monomer which leads to the various physical states of the polymer can be
detected by a change in its deformation temperature. The paper presents the
most popular non-metallic materials used as structural, high-molecular chemical
compounds consisting of numerous elementary units, which represent the same
group of atoms and interconnected chemical bonds. We study the different
physical state of the polymer, can be detected by a change in its deformation
temperature dependence of the deformation is considered graphic, developing
over time at a given voltage, temperature. Considered thermal transitions,
which are among the main characteristics of the polymers, the stress-strain for
linear and cross-linked polymers. Substantiates the average
temperature of the transition regions of not crystallization
linear polymer associated with a change in distance between the material
particles, which in turn, at a
temperature below txr resin becomes brittle, which leads to destruction which is a result of chemical bond in the
macromolecule. Crystalline polymers are considered below the melting
temperature - crystallization tk, which are solid, but they have different
stiffness due to the presence of the amorphous part, which can be in different
states.
Keywords: non-metallic materials, structural materials, physical
and mechanical properties, rubber, polymers, plastics, stress, strain, of the
physical state of the polymer temperature recrystallization.
Introduction
The use
of non-metallic materials provides significant economic efficiency. To improve
the physical and mechanical properties, various additives (ingredients). This
material imparts improved physical and mechanical properties (in comparison
with irregular polymers).
Non-metallic materials - are organic,
inorganic and polymeric materials: different types of plastics, composite
materials on the basis of non-metallic and rubber, adhesives, sealants, coatings,
as well as graphite, glass, ceramics. As constructional materials they are an
important addition to metals, in some cases advantageously replaces them and
are themselves sometimes indispensable.
The
advantage of non-metallic materials are such properties as sufficient strength,
rigidity and elasticity at low density, light transmission, chemical
resistance, dielectric properties, make these materials are often
irreplaceable. Also of note is their adaptability and efficiency in use. The
complexity in the manufacture of products from non-metallic materials in 5-6
times lower, they are 4-5 times cheaper than metal. In this connection, use is
continuously increasing non-metallic materials in mechanical automotive,
aviation, food, refrigeration and cryogenics et al. Engines of internal
combustion ceramics dispense with cooling water, which is impossible in the
manufacture of metal; fairings missiles made only from non-metallic materials.
It is hard to household utensils, audio and video equipment, computers, sports
equipment, cars and other equipment without non-metallic materials - plastics,
laminates, ceramics, rubber, glass and others.
The
basis of non-metallic materials are polymers, especially synthetic. Creator of
the structural theory of chemical structure of organic compounds is A.M. Butlerov. Industrial production of the first
plastmass - the result of work carried out by G.S. Petrov, S.V. Lebedev
performed the world's first industrial synthesis of rubber, NN Semenov
developed the theory of chain reactions and distributed on the mechanism of
chain polymerization. The successful development of polymer chemistry and
physics associated with the names of prominent scientists: P.P. Kobeko, V.A.
Kargina, A.P. Alexandrov, S.S. Medvedev, S.N. Ushakov, V.V. Korshak et al.
Development of heat-resistant polymers associated with the name K.A. Andrianov.
In the field of polymeric materials contributed greatly to foreign scientists:
K. Ziegler, D. Nutt et al. [1, 2].
Outlined
in this article describes the most popular material non-metallic materials used
as structural. Macromolecular polymers are called chemical compounds consisting
of numerous elementary units are the same group of atoms linked by chemical
bonds. Macromolecules are long chains of monomers, which determines their
greater flexibility. Individual atoms in the monomers are connected together
quite strong covalent chemical bonds. Between the macromolecules of polymers
are much weaker physical connection. Their molecular weight ranges from 5,000
to 1,000,000 With such large amounts of macromolecules properties of materials
depend not only on the chemical composition of these molecules, but also their
mutual arrangement and structure.
The theoretical calculations
The
equations for calculating the physical and mechanical properties of
non-metallic materials have been used to study changes in materials under the
influence of temperature - is a necessary condition for establishing the nature
of many phenomena and theoretical calculation of stresses and cutting forces.
Polymers
are classified according to various criteria: composition, form macromolecules,
phase state, polarity, with respect to heat, etc. By nature, all of the
polymers can be divided into two groups - natural and synthetic. Polymers found
in nature - organic substances of plant and animal origin, as well as minerals.
The synthetic polymers prepared from single substances by chemical synthesis.
The main advantage of synthetic to natural polymers are unlimited supplies of
raw materials and extensive synthesis of polymers with predetermined
properties. Feedstock for synthetic polymers are the products of chemical
processing of oil, natural gas and coal. The thus-obtained low-molecular weight
substances are called monomers. They are processed into polymers during
subsequent chemical processing.
As a
method for producing polymers is divided by polycondensation and
polymerization. Polymerization - process a large number of chemical compounds
in the monomer molecules per polymer molecule without big changes the elemental
composition of the monomer. In the polymerization process there is no release
of reaction byproducts. The elemental composition of polymers and monomers are
identical. Polycondensation - the formation of a polymer from monomers of
different molecules in a chemical reaction with the release of reaction
byproducts. Elementar composition different from the composition of the polymer
participating in the polycondensation reaction of the monomers. Schematically,
the formula of the polymer are recorded in the form of [M] n where M - the
chemical structure of the monomer; n - index characterizing the degree of
polymerization. On the composition of all polymers are divided into organic,
organometallic, in organic. Organic polymers comprise the most extensive group
of compounds. If the main molecular chain of such compounds formed only carbon
atoms, they are called carbon-chain polymers. In heterochain polymers atoms of
other elements present in the main chain, in addition to carbon significantly
alter the properties of the polymer. Thus, oxygen atoms in macromolecules
contribute to the flexibility of the chain, the phosphorus atoms and chlorine
increase fire resistance, gas barrier sulfur atoms attach fluorine atoms,
according polymer high chemical resistance, etc. Organic polymers are resins
and rubbers. Organometallic compounds not found in nature. This class of
materials is completely created artificially. They comprise a main chain
composed of inorganic atoms (Si, Ti, A1) was combined with the organic radicals
(CH3, C6H5, CH2). These radicals are attached to the material strength and
elasticity, and inorganic atoms reported improved heat resistance. Their
representatives are silicones. By inorganic polymers include silicate glass,
ceramic, mica, asbestos. As part of the carbon skeleton of these compounds are
not present. Basis materials are inorganic oxides of silicon, aluminum,
magnesium, boron, phosphorus, calcium, and others. The organic radicals
consisting of inorganic polymers are absent. By inorganic and include polymers,
basic molecular unit which, as in the case of organic polymers, composed of
carbon atoms, such as graphite and diamond, with graphite and contains a minor
amount of hydrogen atoms. However, unlike organic polymers forming the basic
molecular unit preferably in the form of linear chains, graphite and diamond
form spatial structures. This gives them properties dramatically different from
the properties of organic polymers. Graphite is the only material remaining in
the solid state at a temperature above 4000 ° C and the diamond is the hardest
substance [1, 2].
Test data
Various
physical state of the polymer change detected by its deformation temperature.
Graphic dependence of the strain that develops over time at a given voltage,
the temperature is called thermomechanical curve (Figure 1) [3].
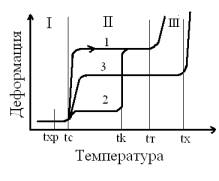
Deformation/Temperature
Figure
1 Thermomechanicalnoncrystalline linear curves (1), the crystalline (2) and rarely mesh
(3) Polymer (tc, tk, ty, tx - Tg, crystallization began and the beginning of
the viscous flow of the chemical decomposition, respectively), I-III - portions
glassy, highly elastic and viscous state
On the
curves are three sections corresponding to the three physical states. The
average temperature of the transition regions are called transition
temperatures. Linear nekristalliziruyuschegosya polymer (curve 1), the region I
- Region of elastic deformations associated with the change in the distance
between the particles of matter. At temperatures below thr polymer becomes
brittle. Fracture occurs as a result of rupture of chemical bonds in the
macromolecule. In region II, the small voltage causes movement of the
individual segments of macromolecules and their orientation in the direction of
the force. After removal of the load molecules as a result of intermolecular
forces take the initial equilibrium shape.
Rubbery
state is characterized by significant reversible deformations. Near the point
ty except elastic and rubbery deformation occurs and plastic. Crystalline
polymers below the melting temperature - crystallization tk - are solid, but
have different stiffness (Figure 1, curve 2) due to the presence of the
amorphous part, which can be in different states. When tk-crystalline portion
melted and thermomechanical curve almost abruptly reaches 1 part of the curve
corresponding to the rubbery deformation, as in non-crystalline polymer. Rarely mesh
polymers have thermomechanical type curve 3. mesh nodes prevent relative
movement of the polymer chains. In this connection, when raising the
temperature of the viscous flow does not occur, and extended rubbery region
becomes the upper limit temperature of the chemical decomposition of the
polymer tx. Thermal transitions (tc and CT) are among the main characteristics
of the polymers. The stress-strain for linear and cross-linked polymers are
different. Linear polymers in the glassy state have a certain mobility of the
segments, so the polymers are not so fragile as the inorganic substance. Under
the action of large stresses in glassy polymers developed significant
deformations, which by their nature are close to rubbery. These strains were
named A.P. Alexandrov forced-elastic, and the phenomenon - forced elasticity.
Arte-elastic deformations occur in the temperature range tc - txr, and when
heated above tc they are reversible (Figure 2). The maximum in the curve is
forced to limit flexibility. Polymers with a dense network structure under load
there is elastic and highly elastic deformation, plastic deformation is usually
absent. In comparison with linear polymers of the elastic deformation portion
is a relatively large, highly elastic deformation is much lower. Nature rubbery
deformation as a linear polymer, is a reversible change in the spatial shape of
the polymer molecules, but the maximum tensile strain usually does not exceed
5-15%.

Figure
2. Diagram of stretching: a - a glassy polymer; b - a dense polymer network
structure, I - elastic deformation region; II - rubbery deformation region
For
crystalline polymers, the stress-strain line is expressed with clear
transitions. In the first stage (part I) is proportional to the elongation of
the acting force. Then the sample occurs suddenly "neck", whereupon
the elongation increases at constant power up to a considerable amount. At this
stage, the cervix (section II) is lengthened by a thicker part of the sample.
After the entire sample turned into a neck, the process proceeds to the third
stage (part III), ending rupture. The structure and properties of the material
different from that of the neck and the properties of the original sample: the
elements of the crystal structure are oriented in one direction (recrystallize)
[3].
Literature:
1. J.M.
Lahtin, V.P. Leontiev. Materials science.M.: Engineering, 1990.
2. Edited
by S.I. Bogodukhov, V.A. Bondarenko. Technological
processes of engineering production. Orenburg,OSU,
1996.
3. Source:
http://5fan.ru/wievjob.php?id=6006.