Tåõíè÷åñêèå íàóêè/ Ìåõàíèêà
Banshidhar Choudhary1, Nickolay Zosimovych2
1Sharda University (Greater Noida, India)
2Instituto Tecnólogico de Monterrey (Campus
Gualajara, Mexico)
EXPERIMENTAL INVESTIGATIONS
OF COMBUSTION PROCESSES OF HCM
In the report has been analyzing experimental researches of processes the
combustion of aluminum agglomeration heterogeneous condensed
mixtures. Heating of agglomerates on the burning surface and
agglomerates, detached from the surface of the combustion happens in an oxidizing
atmosphere, and always ends with their inflammation and combustion. Heterogeneous
mode of burning of agglomerates connected with diffusion of gaseous oxidizer to
the surface of the particle and the chemical reaction of oxidation on the
surface. This results to the formation of the surface layer of aluminum oxide
agglomerate.
Keywords: Heterogeneous condensed mixtures (HCM), composite,
solid rocket fuels, combustion, burning surface, agglomerates, aluminum oxide, modes
density function (MDF), mass fraction, concentration of metal, ![]() phase.
phase.
Introduction. Heterogeneous condensed mixtures (HCM) are
condensed substances capable of self-sustaining combustion and consisting of the powdered ingredients, pressed, and distributed in continuous polymer matrix. The characteristic feature of
heterogeneous condensed mixtures is
great volume content in them
dispersed components that can be reaching 90% or more. Thus, the heterogeneous condensed mixtures are highly
filled composites.
Mixed solid propellants
are complex composite materials which contain the dispersed components,
distributed in a continuous polymer matrix. Dispersed components of mixed solid
rocket propellants are oxidizer (ammonium perchlorate, ammonium nitrate, etc.),
energy supplements, which include powdered explosives such as nitramines (HMX)
and RDX, and powdered metals (aluminum, boron, magnesium, etc.) or their
hydrides. The particle size of dispersed components can vary from a fraction of
a micron to several hundred microns, while the overall mass fraction of
dispersed components in mixed solid rocket fuels can reach 90% [1].
Physical and
mechanical, thermal, ballistic, electrical and other properties of mixed solid
rocket fuels, as highly filled composites, are defined not only by properties
of the components and their content and dispersion, but also the distribution
of dispersed components in the volume of material.
The
ballistic efficiency of solid propellant missiles of different classes
determined first of all, a ballistic efficiency of its propulsion rocket
engines, so, progress in rocket technology for various purposes is largely
related to improving the fuel for solid rocket engine (SRE). Improving SRE is going
in two main directions: improvement of structural materials and the improvement
of solid rocket propellants.
Problem setting. Experimental
researches of processes the combustion of aluminum agglomeration HCM many
papers, which address the impact of various factors on both the distribution of
the agglomerates in size, and the structure and chemical composition of the
agglomerates.
Results and Discussion. Experimentally founded [2-4] that the k-phase
particles in combustion HCM have two modal and even polymodal size
distribution. It is related with two major processes that produce. The original
process is the formation of agglomerates of metal which form coarse fraction
(> 10 mkm) k-phase particles in combustion products.
Heating
of agglomerates on the burning surface and agglomerates, detached from the
surface of the combustion happens in an oxidizing atmosphere, and always ends
with their inflammation and combustion.
Burning
of large aluminum particles (agglomerates) occurs in two modes: the gas-phase
and heterogeneous [5, 6]. Burning in the gas-phase mode connected to
evaporation of the metal and its vapor in the combustion mixture with gaseous
oxidizer. The result of this process is the formation of gaseous the higher
oxides of aluminum, which as a result of chemical condensation constitute fine
particles of aluminum oxide [6]. These particles have a size less than 5-10 mkm
and forming first (highly dispersed) mode condensed combustion products of HCM.
Heterogeneous
mode of burning of agglomerates connected with diffusion of gaseous oxidizer to
the surface of the particle and the chemical reaction of oxidation on the
surface. This results to the formation of the surface layer of aluminum oxide
agglomerate. In addition, the large agglomerates slip around fine particles of
aluminum oxide generated in the gas phase by chemical condensation, which also
leads to an increase in the mass of aluminum oxide which is part of agglomerate
[6]. Thus, the agglomerate is a binary drop of metal oxide and is composed
mainly of unburned aluminum and aluminum oxide which in case of combustion
power plants are liquid. Should be noted that the structure of agglomerates can
also be part products of incomplete decomposition of the organic binder,
primarily carbon [7]. Due to surface tension of aluminum oxide is usually
collected in compact formation (blotches) [6, 8], takes some of the surface of
agglomerate.
Because
normally of agglomerate temperature significantly lower than the temperature of
vaporization of aluminum oxide during evolution the agglomerate is irreversible
accumulation of aluminum oxide and reducing the share of pure aluminum in the
agglomerate. In other words, the mass of aluminum oxide in the agglomerate
increases monotonically as long as the will burn out all the free aluminum or
does not stop the burning process of agglomerate. There are theoretical
considerations [6] that in a chemical reaction ![]() (in k-phase sinter), the
formation of gaseous oxides of higher
(in k-phase sinter), the
formation of gaseous oxides of higher ![]() and
and ![]() that should result to a certain decrease in the
weight of
that should result to a certain decrease in the
weight of ![]() in agglomerate. However seems, the process is
slower than the accumulation
in agglomerate. However seems, the process is
slower than the accumulation ![]() in the agglomerate by heterogeneous oxidation of aluminum, and cannot
change the qualitative picture of formation
in the agglomerate by heterogeneous oxidation of aluminum, and cannot
change the qualitative picture of formation ![]() coarse particle fraction.
coarse particle fraction.
Thus the
main modes density function (MDF) on of particle sizes of aluminum oxide in the
HCM combustion products have a simple explanation: MDF first mode connected
with highly dispersed aluminum oxide, formed by the condensation from the gas
phase, while the other modes MDF describe major functions of the particles of
aluminum oxide, caused by agglomeration of metal by burning HCM and
accumulating them ![]() in the heterogeneous reactions with the gaseous
oxidizer.
in the heterogeneous reactions with the gaseous
oxidizer.
Experimentally
obtained [3, 4, 6] that the size of the agglomerates MDF is also a multimodal. This
is due to several processes involved in the agglomeration. For example, there
is a finite probability that part of the source of metal particles will leave
from the burning surface of MDF, does not combined with other particles. Namely
solitary initial metal particles taken off from the burning surface, form the
first mode of MDF agglomerates [6, 9]. Emergence of other modes MDF has not received
satisfactory explanation to the present.
Structure of Agglomerates. Structural study of agglomerates plays an important role
in understanding of the process of metal agglomeration and further evolution of
particles. As we mentioned above, these agglomerates typically include unburned
aluminum, aluminum oxide and products of incomplete decomposition of an organic
binder. In [10, 11] you can find a
description of two qualitatively different types of agglomerates which are
formed during combustion of HCM composed of different particles. In [10] the
authors suggests to discriminate between two classes of HCM (À and Â). HCM belonging to
class A are characterized by burning of metal in the lower part of the frame
layer. Initially, particles burn in the heterogeneous mode and then they burn
in the upper portion of the frame layer and in the vapor phase on its surface [12].
The sizes of aluminum particles which burn in heterogeneous mode are close to
the sizes of primary metal particles in HCM. These mixtures are characterized by
high connectivity of metal particles to carbon elements of the frame layer. As a
result, pores in the carbonic frame shall be filled with liquid aluminum oxide.
Large contact surface between melted aluminum oxide and the carbonic frame
results in low mobility of the agglomerates and caters for strong adhesive
force which keeps them on the burning surface of HCM.
When HCM
class A burns, agglomerates of two types shall be formed. One type of agglomerates
called “matrix agglomerates” by authors [10] is represented by spherical particles
![]() with some integrated particles of aluminum. As a rule, there is a few
large and many small particles among the aluminum particles, which are
generally located on the free surface of liquid oxides, however, some small
with some integrated particles of aluminum. As a rule, there is a few
large and many small particles among the aluminum particles, which are
generally located on the free surface of liquid oxides, however, some small ![]() particles may be fully encapsulated into aluminum oxide. The metal of
"matrix" agglomerates, which has a free surface, burns in the vapor
phase forming a plume with layered structure. The mass fraction of aluminum
oxide in the "matrix" agglomerates may reach 60-70%. The agglomerates
belonging to the second type consist of metal particles with splats of aluminum
oxide on their surfaces. The proportion of the agglomerates of the first and
second type changes as combustion conditions of HCM belonging to class A change.
There is a threshold pressure above which the first type agglomerates are
practically absent. The threshold pressure depends on the properties of HCM. Generally,
the agglomerates of the first type have bigger sizes, and the sizes increase
with increasing pressure.
particles may be fully encapsulated into aluminum oxide. The metal of
"matrix" agglomerates, which has a free surface, burns in the vapor
phase forming a plume with layered structure. The mass fraction of aluminum
oxide in the "matrix" agglomerates may reach 60-70%. The agglomerates
belonging to the second type consist of metal particles with splats of aluminum
oxide on their surfaces. The proportion of the agglomerates of the first and
second type changes as combustion conditions of HCM belonging to class A change.
There is a threshold pressure above which the first type agglomerates are
practically absent. The threshold pressure depends on the properties of HCM. Generally,
the agglomerates of the first type have bigger sizes, and the sizes increase
with increasing pressure.
When HCM
class B burns, ignition of metal particles occurs on the burning surface (after
they leave the frame layer). The upper portion of the frame layer is composed
of primary metal particles covered with a solid oxide film. After ignition, the
particles will be interconnected in cracks of the oxide film, and then burn in
the heterogeneous combustion mode and in the vapor-phase mode [12]. During
combustion of HCM class B only agglomerates of the second type will be formed,
however, splats of aluminum oxide may be absent on aluminum particles in certain
circumstances. The content of ![]() in the agglomerates shall be specified mainly
by heterogeneous combustion on the surface of the frame layer.
in the agglomerates shall be specified mainly
by heterogeneous combustion on the surface of the frame layer.
The mass
fraction of aluminum oxide is different in agglomerates of different types,
moreover, it depends on the pressure at which HCM combustion occurs. The
publication [10] specifies that the mass fraction of ![]() in the agglomerates formed during combustion of
HCM class A decreases with increasing pressure, and when pressure is
in the agglomerates formed during combustion of
HCM class A decreases with increasing pressure, and when pressure is ![]() MPa, it levels off to approximately 0.28. At the same time, the content
of
MPa, it levels off to approximately 0.28. At the same time, the content
of ![]() in the agglomerates formed
during combustion of class B compounds, is practically independent from
pressure, and is approximately 0.16. It must be noted, that in general, data
provided by different authors indicating mass fraction of
in the agglomerates formed
during combustion of class B compounds, is practically independent from
pressure, and is approximately 0.16. It must be noted, that in general, data
provided by different authors indicating mass fraction of ![]() in agglomerates differ. The reason for it is
different sampling procedures used for chemical analysis. The further sampling
is made from the burning surface, the lower the content of active aluminum in
agglomerates [14].
in agglomerates differ. The reason for it is
different sampling procedures used for chemical analysis. The further sampling
is made from the burning surface, the lower the content of active aluminum in
agglomerates [14].
The
complex structure of the agglomerates containing ![]() products of incomplete decomposition of the organic binder and, in some
cases, gas inclusions, results in dependence of the density of agglomerates upon
their sizes, HCM composition and HCM burning conditions, but primarily upon pressure
[10, 13]. In [10] you can read that in HCM class A the average density of the
agglomerates increases with increasing pressure, and when
products of incomplete decomposition of the organic binder and, in some
cases, gas inclusions, results in dependence of the density of agglomerates upon
their sizes, HCM composition and HCM burning conditions, but primarily upon pressure
[10, 13]. In [10] you can read that in HCM class A the average density of the
agglomerates increases with increasing pressure, and when ![]() MPa, it levels off corresponding to weight ratio between
MPa, it levels off corresponding to weight ratio between ![]() and
and ![]() in the agglomerate.
in the agglomerate.
In some
cases, we can observe bloating of agglomerates owing to extensional emission of
gas due to chemical reactions ![]() and
and ![]() [10, 14]. This results in
changed average densities of the agglomerates and also the difference between
the mass median and volume median diameters of agglomerates.
[10, 14]. This results in
changed average densities of the agglomerates and also the difference between
the mass median and volume median diameters of agglomerates.
Influence of Various Factors on the Process of
Agglomeration. The process of agglomeration is influenced by a large number of factors
which can be divided into two groups. The first group includes factors
characterizing HCM itself (composition, structure, dispersion of components,
burning rate, etc.), the second group includes factors characterizing external
conditions under which HCM combustion occurs (pressure, blowing of burning
surface, overloads etc.).
Concentration and Sizes of Metal Particles in
HCM. It was discovered that there is a certain threshold concentration of
metal in HCM, starting from which agglomeration becomes noticeable [15]. The
threshold concentration of aluminum is directly dependent on the particle sizes:
the smaller the particles, the less their concentration at which agglomeration
occurs. Table 1 shows the data from [15], which demonstrate how the threshold
concentration of aluminum changes in HCM depending on the mass median diameter
of primary aluminum particles.
Table 1
Dependence of threshold concentration of aluminum
under which agglomeration starts upon the mass median diameters of primary
particles of aluminum in HCM
|
Mass median
diameter of Al particles, mkm |
|
10 |
50-70 |
160 |
|
Threshold concentration, % |
1 |
2-3 |
5-7 |
10 |
The
agglomeration process may be characterized by the degree of agglomeration equal
to the ratio of the average diameter of agglomerates to the average diameter of
primary metal particles in HCM.
The
transition from finely divided to large particles of aluminum will be accompanied
by increase in the mass median diameter of agglomerates and simultaneous decrease in the degree of
agglomeration [4, 16].
With
growing concentration of aluminum in HCM the mass median sizes of agglomerates
increases exponentially [4].
The
influence of dispersion and concentration of the metal fuel in HCM upon the
sizes of agglomerates can be simply explained [4]: the smaller the particles of
the primary metal and the larger its mass concentration in HCM, the greater
concentration of metal particles in HCM, the higher the number of contact
particles and, consequently, it is more likely that particles will form
agglomerates and a heat wave will pass through ![]() phase of HCM [17].
phase of HCM [17].
Concentration and Sizes of AP and Other
Disperse Components of HCM. With the increase of mass median diameter of particles
of the oxidizer (AP) the degree of agglomeration and mass median sizes of
agglomerates increase as well [4, 11, 18, 19], and the larger the sizes of AP
particles, the larger the agglomerates.
However,
direct relationship between the average sizes of agglomerate particles and AP particles
shall be observed only for relatively coarse AP powders. The publication [6]
presents data demonstrating the increase in the sizes of agglomerates with
decrease of average diameter of AP particles for mixtures containing fine AP
powder (less than 30 mkm). This effect can’t be explained by a
"pocket" model.
Burning Rate. Almost all researchers pointed
to a positive role of HCM burning rate upon agglomeration process: any changes
increasing the burning rate of HCM help to reduce agglomeration of the metal
during combustion of metallized HCM [4, 18, 19]. In fact, it is very difficult
to separate the influence of HCM
burning rate from the influence of other factors (pressure, oxidizer dispersity,
etc.), which in their turn, influence the burning rate.
For
example, it has been established [20] that aluminum agglomeration will be
practically unobserved in mixtures with fine components containing 15-20 %
aluminum and having the combustion rate of 40-50 mm/s. It is hard to say which
fact plays the major role in this case: either increased dispersion of AP, and
hence, reduction in the sizes of "pockets", or increased burning rate
caused by it. In [21] the burning rate of HCM changed due to changes in the
gravitational density of a compressed sample. By changing in this way the burning
rate of HCM from 5.8 to 7.6 mm/s, the volume median diameter of agglomerates
was reduced by one third as well. However, in this case the mass flow rate of
gas from the burning surface (mass burning rate of HCM) varied within a narrow
range between 0.98 and 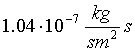 . Thus, decrease in the degree of agglomeration was caused rather by changes
in the structure of HCM, than by the burning rate itself (rate of generation of
combustion gases).
. Thus, decrease in the degree of agglomeration was caused rather by changes
in the structure of HCM, than by the burning rate itself (rate of generation of
combustion gases).
The answer
to the question could be obtained in experiments in which the burning rate
would vary widely due to introduction of catalysts and flame retardants while
chemical and fractional composition of HCM remained unchanged. Unfortunately,
such quantitative data are not currently available.
Role of Fuel and Oxidant. All available data
point to the importance of the kind of the binder and its concentration in HCM.
It is common knowledge that replacement of the binder in HCM under otherwise
equal conditions directly influences the sizes of the agglomerates formed. This
is primarily due to the behavior of the binder when it is heated during combustion
of HCM. At the moment, we have achieved only qualitative understanding of the
role of binder in the agglomeration process: the more carbonaceous residue is
formed and less issuance of the binder volatile under thermal decomposition,
the larger (on the average) sizes of agglomerates [9, 21-24].
For
example in [22, 23], the transition from binders containing 30-40% of the
carbon to binders containing 80-85% of carbon leads to heavy increase by 2-5
times in the average sizes of agglomerates, as well as to qualitative changes
in the dependence of mass median sizes
of agglomerates from pressure.
This is
because of the fact that agglomerates can be bound to the burning surface of
HCM through carbon frame or carbon fibers - products of incomplete decomposition
of the binder. The larger and stronger the carbon residue, the stronger
agglomerates bound to the burning surface. Consequently, the longer they stay
on the surface, the more likely their enlargement.
An important role in the
process of agglomeration plays behavior of the binder and oxidizer when they
are heated. For example, HCM with potassium perchlorate melting during combustion produce
agglomerates smaller than HCM ( other conditions being equal) [6, 25]. The same
applies to the binder: HCM with the binder melting during combustion produces
agglomerates which are smaller compared to agglomerates produced with the
binder decomposing without the liquid phase formation. This behavior can be
explained, presumably, by the fact that the connection with the agglomerates with
a liquid layer on the surface of the HCM is weaker on the average than with
condensed porous frame layer, which contributes to an earlier separation of the
agglomerates that have no opportunity to enlarge on the burning surface.
Accelerations. Experiments show, that normal
accelerations on the burning surface, speed up the agglomeration process [9,
18, 22, 26]. The smaller primary aluminum particles in HCM, the higher the
degree of agglomeration under normal accelerations directed towards the burning
surface. This is due to the increase in time during which agglomerates remain
on the burning surface under the influence of the pressing acceleration which
contributes to enlargement of agglomerates.
Under accelerations
directed towards the burning surface, agglomerate is pressed against the surface
of the binder, thereby accelerating pyrolysis of the binder and, ultimately,
increasing the burning rate of HCM [27-29].
Merging
of agglomerate and aluminum particles protruding to the surface from k-phase, leads to a rapid enlargement of the
agglomerates and contributes to fusion of
agglomerates on the burning surface. As a result, the burning surface shall be
coated with molten aluminum and its oxides.
Increase
of the burning rate of HCM by reduction of AP dispersion of or by introduction of a catalyst enables to reduce
the degree of agglomeration, but, anyway, there are large agglomerates on the burning
surface which are unable to leave it
due to acceleration. The sizes of such agglomerates increase over time, and may
reach several mm.
HCM
Mixing Methods. The end sizes of the agglomerates are influenced not only by dispersion,
chemical and fractional composition of HCM, but also by the method of preparing
a mixture that defines the internal structure of HCM and uniform distribution
of components in it. For example, in [21], the same mixture (10 % PMMA) was
made in two ways: by a conventional mechanical mixing of components in a
rotating drum, when uneven distribution of components is natural, and by
"gelation" when aluminum and oxidant were injected into the
previously prepared dichloroethane solution of propellant.
After
thorough mixing and evaporation of the solvent a relatively homogeneous mass was
obtained in which propellant enveloped aluminum and AP particles with a thin
layer. After combustion of gelled samples containing 7% Al, the average sizes
of the agglomerates were 1.5 times less than in the samples made by the usual
method. This fact also points to the important role of the structure of HCM in
the agglomeration process. However, it should be noted that the burning rate
was slightly higher in the gelled mixture.
Combustion of Aluminum Particles and Agglomerates. Ignition
and combustion of aluminum particles and agglomerates on the burning surface of
HCM is a very important factor affecting the sizes of agglomerates. There is a
large number of publications dedicated to a detailed study of ignition and
combustion of aluminum particles, both single particles and in conditions
relevant to HCM combustion [5, 6, 8-10, 12, 16, 19, 20, 24, 29-33].
Conclusions
In this
paper, the authors considers the basic problems in experimental investigations
of combustion processes in HCM and in their practical application, such as:
1.
Investigation of the internal
structure of heterogeneous condensed mixtures containing disperse components
with different distribution of particles according their sizes by methods of
statistical physics, definition of statistical characteristics describing the
internal structure of HCM.
2.
Analysis of stationary combustion of HCM with consideration of the
statistical structure of its burning surface, development of a statistical
model of stationary-state combustion of HCM.
References
1. Nickolay
Zosimovych, Banshidhar Choudhary. The structure of heterogeneous condensed
mixtures. "Îáðàçîâàíèåòî è íàóêàòà íà XXI âåê – 2012." VIII International Scientific and Practical
Conference, Sofia, 17-25 October, 2012. Collection of scientific
papers on International Scientific Conference, Volume 46. Technologies.
Sofia: Áÿë ÃÐÀÄ-ÁÃ, PP. 51-54, 2012.
2. Fedotova T.
D., Glotov O. G., Zarko V. E. Chemical analysis of aluminum as a propellant
ingredients and determination of aluminum and aluminum nitride in condensed
combustion products. Institute of
Chemical Kinetics and Combustion, Russian
Academy of Sciences, Novosibirsk, Russia, 2002.
3. Glotov O.G.,
Zarko V.E., Karasev V.V., Beckstead M.W. Effect of binder on the formation and
evolution of combustion products of metalized solid propellants. In.:
Combustion and Detonation, 28th Int. Annual Conf. of ACT. Karlsruhe,
Germany, Report 75, 14 p., 1997.
4. Sambamurthi
T.K., Price E.W., Sigman R.K. Aluminium agglomeration in solid-propellant
combustion. AIAA Journal. V. 22, ¹8, PP. 1132-1138, 1984.
5. Brooks
K.P., Beckstead M.W. Dynamics of aluminium combustion, Journal of Propulsion and Power, V. 11, ¹4,
PP. 769-780, 1995.
6. Babuk
V.A., Vasilyev V.A., Malakhov M.S. Condensed combustion products at the burning
surface of aluminium solid propellant // Journal of propulsion and Power, V.15,
¹6, p.783-793, 1999.
7. Êîâàëåâ Î.Á. Ôèçèêî-ìàòåìàòè÷åñêîå
ìîäåëèðîâàíèå àãëîìåðàöèè àëþìèíèÿ ïðè ãîðåíèè ñìåñåâûõ êîíäåíñèðîâàííûõ ñèñòåì,
ÔÃÂ, Ò. 25, ¹ 1, Ñ. 39-40, 1989.
8. Dreizin
E.L. Experimental study of stages in aluminium particle combustion in air.
Combustion and Flame, V. 105, ¹4, PP. 541-556, 1996.
9. Áàáóê Â.À., Áåëîâ Â.Ï., Õîäîñîâ Â.Â. è äð.
Èññëåäîâàíèå ñòðóêòóðû àãëîìåðàòîâ ïðè ãîðåíèè àëþìèíèçèðîâàííûõ ñìåñåâûõ
êîíäåíñèðîâàííûõ ñèñòåì, ÔÃÂ, Ò. 24, ¹5, ñ. 52-57, 1988.
10. Price
E.W., Sigman R.K., Sambamurthi J.K. and Park C.J. Behaviour of Aluminum in
Solid Propellant Combustion. Scientific report. Georgia Institute of technology,
Atlanta, Georgia, 1982.
11. Eggersdorfer
M.L., Pratsinis S.E. The Structure of Agglomerates consisting of Polydisperse
Particles, Aerosol Sci Technol., March, 46 (3), PP. 347-353, 2012.
12. Yang Gan,
Li Qiao. Combustion characteristics of fuel droplets with addition of nano and
micron-sized aluminum particles. Combustion and Flame, 158, PP. 354-318, 2011.
13. Leonid Kaledin,
Frederik Tepper. Metallic Nanopowders: Rocket Propulsion. Dekker Encyclopedia
of Nanoscience and Nanotechnology, second Edition – Six Volume Set (Print
Version), Chapter 189, Metallic Nanopowders, CRC Press, 2008.
14. Babuk
V.A., Belov V.P., Shelukhin G.G. Combustion of Aluminum particles in Composite
Condensed Systems under Low and High Pressures. Leningrad, Translated from
Fizika Goreniya i Vzryva, Vol. 17, pp. 26-31, 1981.
15. Varshney
B.S., Surendra Kumar. Studies on the burning behavior of metal powder fires and
their extinquishment: Part 1 – Mg, Al, Al/Mg alloy powder fires on sand bed.
Fire Safety Journal, Volume 16, Issue 2, PP. 93-117, 1990.
16. Rashkovskii S.A. Effect of
Acceleration on Agglomeration of Aluminum particles During Combustion of
Composite Solid Propellants. Translated from Fizika Goreniya i Vzryva, Vol. 43,
No 6, PP. 40-50, November-December, 2007.
17. Steinman
Robert. Powdered Metals in Modern Life. School of Science and Mathematics, Vol.
41, Issue 2, PP. 131-139, 2010.
18. Cohen N.S.
A Pocket model for aluminum agglomeration in composite propellants. AIAA
Journal. V 21, N5, P.720-725, 1983.
19. Rashkovskii S.A. Statistical simulation of aluminum
agglomeration during combustion of heterogeneous condensed mixtures. Combustion, Explosion, and Shock Waves, 2, 2005.
20. Caveny
L.H., Gany A. Breakup of Al/Al2O3 agglomerates in accelerating flowfields, AIAA
Journals, Vol. 17, ¹ 12, PP.1368-1371, 1979.
21. Ïîõèë Ï.Ô., Áåëÿåâ À.Ô., Ôðîëîâ Þ.Â., Ëîãà÷åâ Â.Ñ., Êîðîòêîâ À.È. Ãîðåíèå ïîðîøêîîáðàçíûõ ìåòàëëîâ â àêòèâíûõ ñðåäàõ, Ì.: Íàóêà,
294 ñ.,1972.
22. Ãðèãîðüåâ Â.Ã., Êóöåíîãèé Ê.Ã., Çàðêî Â.Å.
Ìîäåëü àãëîìåðàöèè àëþìèíèÿ ïðè ãîðåíèè ñìåñåâûõ êîìïîçèöèé, ÔÃÂ, Ò. 17, ¹ 4,
Ñ. 9-17, 1981.
23. Áàõìàí Í.Í., Áåëÿåâ À.Ô. Ãîðåíèå ãåòåðîãåííûõ
êîíäåíñèðîâàííûõ ñèñòåì, Ì.: Íàóêà, 227 ñ.,
1967.
24. Rashkovsky
S.A. Simulation of composite explosives statistical structure. In: Proceeding
of Eleventh symposium on Chemical Problems, Connected with the Stability of
Explosives, Bastad, Sweden, PP. 17-18, 1998.
25. Babuk
V.A., Dolotkazin I.N., Sviridov V.V. Simulation of Agglomerate dispersion in
Combustion off Aluminized Solid Propellants. Combustion, Explosion and Shock
Waves, March, Vol. 39, Issue 2, p.195-203, 2003.
26. Brundige
W.N., Caveny L.H. Low burning rate aluminized propellants in acceleration
fields, AIAA Journal, 1984, V. 22, ¹5, PP.638-646.
27. Richard
Nakka. Effect of Chamber Pressure on Burning Rate for the Potassium Nitrate –
Dextrose and Potassium Nitrate – Sorbitol Rocket Propellants, Issue 1, 1999.
28. Tim Ojala,
Matti Pietikӓinen, Jarkko Nisula. Determining Composition of Grain
Mixtures by Texture Classification based on Feature Distributions. OULU,
Finland, 2009.
29. Rashkovsky
S.A. Ultra-Fine Aluminum Behavior in Composite Solid Propellants combustion.
Proceedings of the European Combustion Meeting “ECM2003”, Orleans, France,
2003, PP. 50/1-6.
30. Massa, L., Jackson, T.L., Buckmaster, J., and Campbell, M. The Three –
Dimensional Combustion of Heterogeneous propellants. 38th JANNAF
Combustion Meeting, Destin, Florida, April, 2002.
31. Waesche
R.H.W., Wenograd J. Calculation of Solid-Propellant Burning Rates from
Condensed-Phase Decomposition Kinetics. Combustion, explosion, and Shock Waves,
Vol. 36, ¹1, 2000.
32. Robert C.
Brown, M. Robert Dawson, Jerod L. Smeenk. Bed Material Agglomeration During
Fluidized Bed Combustion, Work performed under Grant No. DE-FG22-92PC922530,
Iowa State University, 32 p., 1996.
33. Nickolay
Zosimovych, Banshidhar Choudhary. The Structures of Heterogeneous Condensed
Mixtures. PARIPEX - Indian
Journal of Research, Vol. 3, Issue: 4, PP. 135-141, May, 2013.