Shatabaeva
E.O., Urkimbaeva P.I., Burasheva. G.S., Mun G.A.
Kazakh
National University named after al-Farabi, Kazakhstan
Development
of Hydrogelic Dressings Formulation Containing Biologically Active Additive
In
article the development of the formulation of hydrogel dressings containing
biological active food supplements for the treatment of burn wounds, and the
results of the research.
The skin is one of the important components of the
human body and the protective coating from the influence of human and
environmental factors. Skin disorders are widespread. One of the most common
worldwide diseases of the skin - it burns. Burn - is damage to body tissues
caused by the action of heat or the action of certain chemicals.
Burns - one of the most common traumatic injuries in
the world. In general, in the Republic of Kazakhstan annually register
approximately 17,000 patients with burn injuries. Every year about 200 people
die from burn injuries.
Currently the most important effective way of treating
disorders of the skin - the use of objects and materials to protect the
affected area and to facilitate a cure. Close to the ideal treatment for
damaged skin burns and other injuries they released transplantation of skin
from other parts of the body of the same patient. However, it should also
consider limiting factors such as location of the donor site, where you can
take the graft with no harm to the body, as well as the area of the
wound surface, and consequently, the amount of skin needed for transplant. That
is a way of such a method is not always effective. Therefore, for the healing
of the skin using a combination of materials is not harmful to the skin covering.
But in the world of medical practice is still used cotton and bandages (gauze,
cotton). But the sorption properties of these materials are very low, for
example, sorption properties of cotton materials are 14 g / g. Dressings based on polymeric hydrogels can
be used on burns of various degrees due to their special properties. They are
soft, transparent, painless, and coincide with the structure of blood lymph.
Therefore, the use of hydrogel dressings in clinical practice is very
convenient and effective.
Along with the synthetic, of great importance in
medical practice are drugs of plant origin. Suffice it to say that the World
Health Organization needs 80% of the world's population in the primary health
care provided by traditional medicine, including the use of extracts or active
components of plants. Especially in recent years, a tendency toward increased
use of herbal medicines, which is an advantage in their safety and high
selectivity of action. This is facilitated by the presence of the same powerful
raw material base of Kazakhstan.
The purpose of this paper is to develop a formulation
of radiation-chemical method hydrogel dressings on the basis of «alhidin"
and polyvinylpyrrolidone. An important feature of high polyvinylpyrrolidone is
its high adsorptive capacity and tendency to form complexes. High molecular
weight polyvinyl binds many substances, including drugs, toxins, organic and
inorganic pigments, etc. Alhidin is a biologically active complex of camel
thorn (Alhagi Kirgisorum Shrenk), containing polymeric proanthocyanidins,
polysaccharides, flavonoids, amino acids, trace elements and has a broad
spectrum of action: anti-inflammatory, hepatoprotective, wound healing, immune
stimulating, astringent, and other properties.
The active principle of the drug is polymeric
proanthocyanidins. Also, we used a microbiological agar-agar as a cross linking
agent and polyethylene glycol (PEG) to improve the mechanical properties of the
dressing. For the synthesis of hydrogel dressings, in a glass of 250 ml was
added an aqueous solution of PVP, dissolved agar-agar, polyethylene glycol,
respectively, and the desired concentration of the drug "Alhidin."
The resulting mixture is thoroughly mixed, packed in forms, an indoor film.
Each form is placed in a sealed envelope of polyethylene film. Samples were
sent an electronic accelerator ELV-4 located at the Institute of Nuclear
Physics for radiation cross linking of the hydrogel.
In this paper we have investigated the condition of
immobilization of the drug in a polymer gel. Since the drug "alhidin"
is a complex containing several components, the sorption method of
immobilization is not suitable for the occasion. Because, as the sorption
capacity of components àre different and the introduction of these components into a gel in the
same quantities is almost impossible. Therefore, the only effective way is to
introduce the drug in the polymer gel composition method, with the remains of
the original drug.
It can be assumed that the binding of the drug with
agar - agar is due to hydrophobic interactions between hydrocarbon radicals and
intermolecular hydrogen bonds -OH, C = O, NH2 groups of agar-agar and
polysaccharides, proanthocyanidins, and amino acids that make up the drug.
Complex formation between the agar and a drug becomes
noticeable at higher temperatures. Thus, at a temperature of 320 K by adding
the drug there is a noticeable decrease in the viscosity of the system,
indicating that compaction of the polymer chain by complexation with the drug.
This can be explained by increased hydrophobic interactions, leading to the
formation of the complex.
As mentioned above, the drug "alhidin" is a
complex and major components of the drug, depending on the molecular weight are
as follows:
- polimerl³k proanthocyanidins;
- flavonoids;
- polysaccharides;
- amino acids. We have investigated the drug release
pattern of the resulting hydrogel dressings, by paper chromatography. Because
amino acids and a molecular weight polysaccharides below they are the first
released from the hydrogel dressing.
As a result, it was determined that the temperature at
38±50C and 30-45 minutes of starting the release of amino acids and
polysaccharides of hydrogel dressings.
Also, we have investigated the kinetics of swelling
obtained hydrogels in water and saline. In Figures 1 and 2 show the kinetics of
swelling of hydrogel dressings containing 5% and 7% of the concentration of the
drug.
The results show the degree of swelling of hydrogels
in NaCl solutions in more than water.
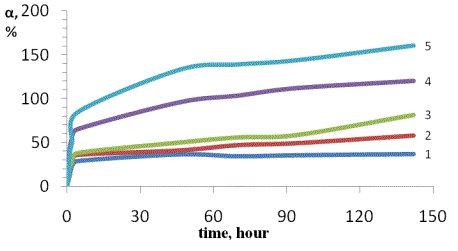
[NaCl],% = 0,45 (2), 0.9 (3) 1.8 (4) 2.7 (5). In water
(1)
Figure 1. Kinetics of swelling of the hydrogel
containing 5% concentration in water and saline
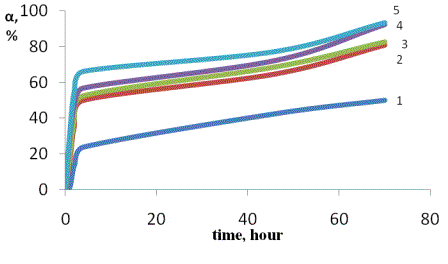
[NaCl],% = 0,45 (2), 0.9 (3) 1.8 (4) 2.7 (5). In water
(1)
Figure 2. Kinetics of swelling of the hydrogel
containing 7% concentration in water and saline
Also, while the swelling of hydrogels are shown in
table 2.
Table 2. Time of swelling of hydrogel dressings
|
[Alhidin], % in a sling |
Swelling time,
days |
|
|
In
water |
In saline |
|
|
5 |
6 |
6 |
|
7 |
3 |
2 |
As can be seen from the table during the swelling of
hydrogels containing 7% of the drug "alhidin" low, because,
especially in saline complexation so much that the complex is released in the
form of flakes (figure 3).
|
|
|
|
à |
b |
|
|
|
|
c |
d |
[Alhidin], % = 7;
Time, hour = 4 (à); 6 (b); 24 (c);
40 (d).
Figure
3. Changing external conditions depending on the time
Thus, the development of technology-based dressings
polymeric hydrogels allows you to implement in medical practice, a new method
for effective and reliable treatment of burn wounds of varying degrees. These
hydrogel dressings more effective than other materials in the treatment of
large wounds.
References:
1.
Shtilman M.I. Polimery mediko-biologicheskogo naznacheniya (Polymers of
bio-medical application, in Russian), M.: "Akadem-kniga",2006, p. 400
2. Burasheva G.S., Rakhimov K.D.,
Abilov J.A. Biologicheski aktivnyi
complex – alhidin i ego farmakologicheskaya aktivnost` (Biologically active
complex - alhidin and pharmacological activity, in Russian), Almaty: 2001, p.
180