Химия и химические
технологии
/5.Фундаментальные
проблемы создания новых материалов и технологий
DPhil, professor, Chumak
O.P., master, Usichenko O. V.
National Technical University “ Kharkov polytechnic institute”,
(Ukraine)
Surfactantes - monoacilglycerines and diacilglycerines
glicerolis
Synthesis the MAG and DAG from fatty acids and glycerin with use lipases Candida Antarctica (Novozim 435).
Rational conditions of synthesis Have been positioned.
Синтез МАГ и ДАГ из
жирных кислот и глицерина с использованием иммобилизированной липазы Candida
Antarctica (Novozim 435). Установлены
рациональные
условия
синтеза.
Monoacilglycerines (MAG) and diacilglycerines (DAG) are the important
emulsifying agents of the edible class, used as those or after the further chemical
modification. Monostearin is used as the additive to edible, cosmetic and
pharmaceutical products.
The traditional chemical method used in the industry for reception the
MAG, consists in glycerolisis of fats and oils at heats (220-260ºС) and increased pressure with use of inorganic alkaline catalysts. The
lack of this method consists in high consumption of energy as its subsequent
distilling is necessary for reception of a qualitative product.
Actual problem is synthesis of
MAG and DAG without use of chemically toxic catalysts and heats. This
problem dares by application enzymatic technologies.
Synthesis the MAG with use of enzymatic processes has a number of
advantages, such as soft conditions of reaction, high selectivity and small
energy consumptions. Enzymatic way of receive of MAG are widely studied now.
Among them it is possible to evolve selective hydrolysis, etherification or
interesterification of fatty acids and glicerolisis of oils and fats.
In [1] the mixture of MAG and DAG
has been received by means of enzymatic hydrolysis triacylglycerins. Differences
in the ratio components has been carried to differences in enzym's
regioselectivity and hydrophilicity of the carrier. In a source [2] it has
described glicerolisis of TAG in which MAG and DAG have been received from hydrogenated
the beef fat and glycerin in the presence of lipase Pseudomonas. The
main product yield depends on temperature of reaction. Berger [3] describes
reception of DAG by means of reaction of etherification of fatty acids and
glycerine in organic solvent. Reaction was made in n-hexane, diethyl ether or
butyl-methyl ether. Also the 2-MAG have been synthesized in a microemulsion of
oil with use anionic surfactants and 1,3-specific lipases [4]. Authors do a
conclusion, that their method is result to a low yield of a product of
synthesis by the MAG from glycerin and fatty acids because of migration acyls.
In [5] the receiving of acylglycerins
from fatty acids and glycerin with use immobilized lipases in the anhydrous
environment is described. Immobilized lipase catalyzed a reaction of synthesis between glycerin and
oleinic acid without use of organic solvents. As a result of reaction it was
received more ТАG, than the MAG and DAG.
Presently a method of synthesis the MAG and DAG which would high yield
of a product at rather low temperatures is necessary. The work purpose of the
work is a detection of factors at which a yield of the MAG and reaction rate
there would be the greatest.
In researches acylglycerins have been received by a method of synthesis
from fatty acids and glycerin with use immobilized lipases Candida
Antarctica (Novozim 435).
Such fatty acids were used: myristic, stearinic and palmitic. These are
saturated acids with the long chain and even quantity of atoms of carbon.
Tetradecanoic acid has 14 atoms of carbon, its molecular weight is 228,36, the
fusion temperature 54,40ºС, the neutralization number (NN) is
245,68. Palmic acid: 16 atoms of carbon, its molecular weight is 256,42, the
fusion temperature is 63,1 ºС, the neutralization
number is 231,46. Stearic acid: 18 atoms, its molecular weight is 284,47, the fusion temperature is 69,6 ºС, the neutralization number is 197,23.
For synthesis of the MAG a molar parity acid : glycerine it is accepted
1:1, temperature of reaction - 60-80 ºС. Quantity of the enzyme
- 5-10 % from weight of acid.
As ester synthesis creates one mole of water for each mole of received
ester for reception of high yield of a product it is necessary to delete water
from a reaction mixture continuously. Therefore we made reaction in vacuo.
Depth of vacuum was 0,8-0,9 from the atmospheric.
The
changing of acid number of reaction showed reaction’s course of reaction mixture. Reaction stopped, when acid number
did not change.
Thus,
it has been received three products - esthers of myristic, stearinic and
palmitic acids.
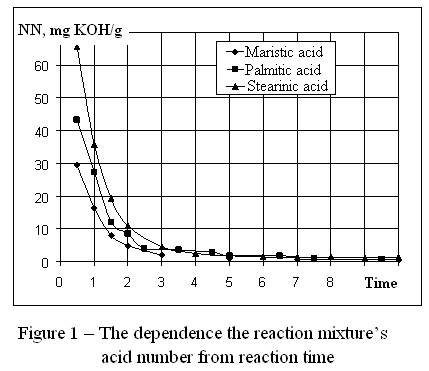 On fig. 1 the dependence of change of acid number of a mixture reactions
is represented eventually. Apparently, reaction passed most intensively at
first two hour. During this period concentration of acids sharply decreased.
With increase in time of reaction, its speed decreased. Apparently from
drawing, there has fast passed reaction of formation of an ether of
tetradecanoic acid.
On fig. 1 the dependence of change of acid number of a mixture reactions
is represented eventually. Apparently, reaction passed most intensively at
first two hour. During this period concentration of acids sharply decreased.
With increase in time of reaction, its speed decreased. Apparently from
drawing, there has fast passed reaction of formation of an ether of
tetradecanoic acid.
Finished products have been analyzed by means of a thin-layer
chromatography.
Simultaneously these products have been analyzed by a chemical method.
For definition of quantity the MAG in a mix was used a method periodic
oxidations. At first the product sample dissolves in ethanol to define, whether
are present at product ТАГ (deposit sediment). In experiences in one of products of etherification
ТАG it was revealed not. Then the emulsifying agent sample dissolves in diethyl
ether and 5 % acetic acid water solution are washed out by. Sample an aqueous
and etheric phase. Periodic solution adds to the samples, maintain within 30
minutes, and then add a solution of potassium iodide and titrate by solution of
sodium thiosulfate before full disappearance
of colouring in the presence of the starch indicator. Then define concentration
of the MAG in ether layer. The same method define concentration of glycerin in
an aqueous phase.
Knowing the maintenance the MAG and glycerin in resultants of reaction,
it is possible to calculate maintenance DAG.
Results of chemical analysis of ethers are resulted in tab. 1
|
Fatty acids,
applied to synthesis |
The content of
the MAG, % |
The content of
the DAG, % |
The maintenance
of glycerin, % |
|
Myristic acid Palmitic acid Stearinic |
37,4 35,6 35,5 |
61,5 63,4 61,1 |
1,1 1,0 1,4 |
Reference:
1.Plou, J.F., Barandidrn, M., Calvo, V.M.,
Ballesteros, A., and Paster, E. (1996) High-Yield Production of Mono -
and Di-oleylglycerol by Lipase-Catalyzed Hydrolisis of Triolein//Ensyme Microb.
Technol. 18, 66-71. 2. Yamane, T.,
Kang, S.T., Kawahara, K., and Koizumi, Y. (1994) High-Yield DAG
Formation by Solid-Phase Ensymatic Glycerolysis of Hydrogenated Beef Tallow//JAOCS
71,339-342. 3. Berger, M., Laumen, K.,
and Schneider, M. (1992) Ensymatic Esterification of Glycerol I.
Lipase-Catalazed Synthesis of Regiosomerically Pure 1,3-DAG//JAOCS 69, 955-960.
4. Homberg, K. and E. Osterberg, (1988) Enzymatic Preparation of Monoglycerides
in Microemylsion//JAOCS 35. 5. Ergan,
E., Trani, M., and Andre, G (1990) Prodyction of Glycerides from
Glycerol and Fatty Acid by Immobilazed Lipase in Non Aqueous Media//Biotechnol.
Bioeng. 35, 195-2000.