Òåõíè÷åñêèå íàóêè
Ìåòàëëóðãèÿ
Zhiguts Yu.Yu.,
Legeta Ya.P., Petrov O.P.
MATERIALS
GOT WITH THE USE OF SHS
Introduction.
SHS has been lately introduced into science and technology by A.G.Merzhanov and co-workers as a means of obtaining
refractory high-hard alloys and phases by using the chemical heat as a result
of synthesis.
Very high burning temperatures (several thousand degrees Centigrade) can
be attained by combining aluminothermic reactions
(producing elemental metals – Fe, Cr, V, Mo, W, by reducing their oxides with
the aid of Al powder) with the oxygen-less burning of these metals in carbon
powder. Such combined reactions were called “hybrid" processes [1]. Medium
high heat evolution processes have been used for hard-fasing
of castings with carbides and /or borides surface layers by the in-mould route
[2]. In the present work height heat evolution processes have been employed in
the production of medium size cutting tools in order to compensate high heat
losses because of rather small volumes of reacting powder mixtures and also in
order to simultaneously weld the carbides metal obtained on the steel holder of
tools.
Theory
and experimental.
The “hybrid” high temperature processes devised can be described by the following
reactions:
Stage 1 (thermit
process − Eq.
1) and stage 2 (oxygen-less burning Eq. 2):
(Me’+Me”)
Me’+nC®Me’Cn+Q2
(2)
Total: (Me’+Me”)
Here Me’ is the
carbide-forming element (e.g. W) and Me” is the metal that is not combined with
carbon but forms the plastic matrix which binds together the hard carbides Me’Cn.
In usual carbide alloys Me” is cobalt. In “carbidostal” [3]. Co is replaced by alloyed tool steel, e.g. 12% Cr or high-speed stell (HSS). In Eq. 1 and 3 the ratio Me’/Me” is not specified. This ratio is very important because it
influences the total heat evolution Q=Q1+Q2
and also the ratio carbidic phase/cobalt or tool
steel matrix in the carbidic alloy or in “carbidostal”. In case of the synthesis of “carbidostal” surplus carbon must be added to the powder
mixtures as per Eq. 2 because this extra amount is
necessary to carburise the austenite+martensite metal
matrix. When this matrix is alloyed with Cr
or with W+Cr
the dissolution of carbon in the liquid iron-based highly hard alloyed solution
gives a small additional evolution of heat Q3
which supplements the sum Q1+Q2.
A programme for computer aided calculations of Q1, Q2
and Q has been elaborated and has
been used in ref. 3. In fig. we have shown the microstructure of "carbidostal"
and of its steel matrix.
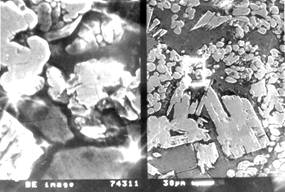
Fig. Microstructure
of a "carbidostal" produced by the SHS (self-propagating
hightemperature synthesis): a − massive WC carbides in an ultra high-carbon
high-tungsten steel matrix (magnification x100); b − matrix with spheroidized complex carbides (magnification x400)
This "carbidostal" has been obtained
by the "hybrid" process using Fe2O3,
Cr2O3, WO3 graphite and aluminium
powder mixtures. Tungsten binds most of the carbon into large WC carbides, shown in fig. 1. Another
part of tungstem binds carbon into small WC carbides forming the complex eutectic
matric. This matrix may also contain small amounts of
other carbides: Me7C3,
Me6W6C2
et al. In fig. we can see that these eutectic carbides have been strongly spheroidised during rheocasting,
when sharp edges have been rounded up. The investigate
this alloys (at the x-rey spectrum of this alloy obtained in CuKa radiation) are
shows only the WC and W2C carbide phases seen in
it. The iron-rich phase is a'-martensite obtained after self-quenching and triple
tempering at 5700C. The hardness of the alloys obtained in such a
new way is 72-75 Ra. Medium size
cutting tools have been obtained by burning thermit+SHS
powder mixtures in a highly refractory combustion chamber placed over a small
refractory mould surmounting the preliminarily heated steel holder of the tool.
The combustion chamber and the mould must be separated by a thin titanium
sheet. The exothermic mixture is ignited by a small amount of Mg or Ti powder, which is ignited itself by an ordinary match. When
burning reactions end the slag floats up and the extremely hot liquid phase
burns through the titanium sheet and fills the ceramic mould, being thus
automatically welded to the steel holder of the tool. Such technology excludes
brazing and other operations, designed to join the carbide alloy to steel. A number of
different types of tools for metal cutting and rock boring have been produced
in such a novel way with good exploitation features in semi-industrial and
laboratory conditions. The further work must be focused on augmenting the
content of primary WC carbides in the "carbidostal"
obtained, the partial replacement of WO3
by TiO2 and other subjects
of investigations.
TiO2
is much less prone to oxidise Al than
WO3, the thermit-type reaction being much less exothermic in case of
replacement of W by Ti. Therefore such full replacement is
impossible. Yet the SHS reaction Ti+C=TiC is very "hot", the adiabatic temperature
of such an oxygen-less burning being 3200 K.
More exact computations and experiments must reveal the extent of partial
replacements of that type.
References:
1. Æóêîâ À.À., Ìåðæàíîâ À.Ã., Áîðîâèíñêàÿ È.Ï.
Èñïîëüçîâàíèå ÑÂÑ â ëèòåéíîì ïðîèçâîäñòâå//Ëèòåéíîå ïðîèçâîäñòâî. M:.
Ìàøèíîñòðîåíèå. 1984, ¹ 11. − Ñ. 2−3.
2. Æèãóö Þ.Þ., Æóêîâ À.Î. Íîâ³òí³
òåõíîëî㳿 âèãîòîâëåííÿ òà çì³öíåííÿ äåòàëåé ³ç âèêîðèñòàííÿì ÑÂÑ-ïðîöåñ³â// Âîñòî÷íî-åâðîïåéñêèé
æóðíàë ïåðåäîâûõ òåõíîëîãèé. − Õàðüêîâ. − Òåõí. Öåíòð. −
2007. − ¹1 (25). − Ñ. 32−38.
3. Zhiguts Yu.Yu., Shurokov
V.V. Carbide steels synthesized by metallothermy// Materials Science.
Springer. New York. − 2005. − V. 41. ¹5. − P. 666−672.