ÓÄÊ 621.7\9 ¹8 Îáðàáîòêà
ìàòåðèàëîâ â ìàøèíîñòðîåíèè
Ê.ò.í. Cîøêî Â.À.
Õåðñîíñêèé
íàöèîíàëüíûé òåõíè÷åñêèé óíèâåðñèòåò
Hydrogen permeation into iron and steel
during plastic deformation
Recently there has been increasing
interest in the study of hydrogen permeation into metals preliminarily
subjected to plastic deformation [1]. Most work in this area has concerned the
investigation of hydrogen diffusion into iron and steels plastically deformed
right: before diffusion experiments. The peculiarity of such diffusion process
is specific dependence of diffusion coefficient on plastic deformation degree
[2]. The mechanism of the process is usually treated in terms of creation of
hydrogen traps (dislocations, vacancies, grain boundaries etc.) in metal
crystalline lattices, for bcc metals or alloys binding energy of hydrogen atom
to trap being much more in comparison with activation energy of diffusion [3].
By now hydrogen diffusion into metal
directly during its plastic deformation (in situ) is not widely studied due to
experimental difficulties [4]. At the same time this problem is extremely
interesting from both theoretical and applied points of view, covering wide
range of phenomena from thermodynamically nonequilibrial mechano-chemical processes to the
effect of hydrogen containing media on
the wear of machine elements during friction, cutting fluids efficiency in
metal working etc.
The object of this paper is to present
the preliminary results of investigation of hydrogen permeation into iron and
steel during plastic deformation in hydrogen containing media: water,
hydrocarbons etc.
Methods of hydrogen
detection
Several methods of hydrogen detection
in metals are described in literature:
1. Permeation of hydrogen into metals,
which are capable of crystal hydrides formation (titan, nickel etc.) may be detected
directly by crystallographic methods
[1,5].
2. Kinetic or steady state
measurements of hydrogen permeation through thin metal membranes [6,7].
3. Nuclear physics methods, including
tritium irradiation measurements and recently developed approach based on
reaction of helium-3 atoms preliminary implanted into metal with deuterium
atoms diffused through metal and trapped by defects created around helium
nuclei [8,9].
4. The most widely spread method uses
simple measurement of gas evolution from the sample at heating. It gives
usually only the total amount of hydrogen in the
sample but not its spatial distribution or energetic characteristics [10,11].
Method of gas evolution was modified
in our work by applying the idea of thermo-desorption spectroscopy (TDS)
usually used in surface science investigations [11]. The sample was heated in
vacuum at a rate of 0.5 K/sec. with continuous mass-spectral analyses of the
gas phase. Experimental curves present the dependence of mass-spectral signal
on the selected mass (proportional in our case to the rate of hydrogen or
deuterium evolution) on temperature of the sample. Usually the curve
(TDS-spectrum) has one or several peaks with maximal according to temperature
of evolution of hydrogen of different "types". The
positions of maximal on the curve as well as the peak shapes reflect the
complex process proceeding in the case of linear temperature raises, the main
components of which are the diffusion of hydrogen atoms through the metal,
their exit and recombination on the surface leading to molecular hydrogen
evolution. It is clear that the distribution of hydrogen along the sample
before heating as well as the typical size of the sample determine form and
positions of the peaks.
Two kinds of plastic deformation -
compression and cutting were studied.
Plastic deformation
during compression
The fact of
hydrogen permeation into metal was established in the experiment of compression
the iron sample in D2O. The cubic sample (1=3mm) was placed between
parallel platens inside D2O drop. After load of 20 tons the sample
was compressed to thickness of 0.5 mm (relative deformation of compression
80%). Then the surface layer of 50-mu thickness was grind from both sides of
the sample in order to remove possible surface compounds containing deuterium.
Using of marked water enables to avoid detection of "outside"
hydrogen already present in the initial sample or introduced from atmospheric
water during grinding. Peak of deuterium evolution with maximum at 540 K was
found in the TPD spectra of compressed and grind sample, thus proving its
permeation into iron (see Fig. 1).

Fig.1
Plastic deformation during cutting
Main parts of the experiments were made with cutting (drilling), which is accompanied by high-speed plastic deformation of the treated material. Cutting was made in different medias listed in the table. Cutting of the sample presaturated with hydrogen by electrochemical method was also made. The same amount of chips (50 mg) was taken for each TPD experiment after cutting.
Hydrogen peaks position
and corresponding time of sample exposure at room temperature (texp)
before TPD beginning are tabulated in the Table.
Hydrogen peaks position
in TPD spectra.
NO Media texp T max (oÊ)
___________________________________________________________________
1 air 0.5 480
2 dry
nitrogen 0.5 -
3 H2O 0.5 425
4 H2O 2 425
5 H2O 23 493
6 H2O 300 -
7 H2O 1
(=70 C) 490
8 D2O 0.5 425
9 D2O
(large fraction) 7 480
10 D2O
(small fraction) 7 490
11 D2O
(large fraction) 50 505
12 D2O
(small fraction) 50 505
13 electrolysis 0.5 475
14 electrolysis 29 480
15 ethanol 0.5 480
16 ethanol
+ water 0.5 443
17 n-heptane 0.5 455, 505
18 vaseline oil 0.5 455,
505
19 oleic
acid 0.5 500
20 vaseline oil + water 0.5 425, 480
___________________________________________________________________
Note: 0.5 hour – minimal time for preparing the TPD
experiment.
1. Cutting in water
TPD spectra received 0.5, 2, 23 and
300 hours respectively after cutting in water are shown in the Fig. 2. Maximum
of the TPD peak of fresh chips is located at lowest temperature and has largest
integral intensity. The total amount of hydrogen penetrated into chips is
estimated to be of the order of 3-10 ppm.

Fig.2
With increase of Lime the peak maximum
shifts to higher temperature with simultaneous lowering of intensity reaching
zero after several days. If the chips are kept at higher temperature before TPD
experiment then hydrogen redistribution accelerates and the peak shift to
higher temperature need much less time.
To exclude the possibility of
registration the "outside" hydrogen in TPD spectra cutting in D2O was also
made. The results were the same (see table).
In order to estimate the influence of
chips size on the peak position the TPD experiment for two different chips
fractions (sieve size - 0.3mm) was made. It is seen from the table that the
particle size does not influence noticeably the peak position for time of
exposure of 7 and 50 hours.
The dependence of peak maximal
position in TPD curve after cutting in water on time of exposure is shown in
Fig. 3. The dependence is asymptotic; the temperature of peak maximum reaches
its limit at approximately 6 hours.
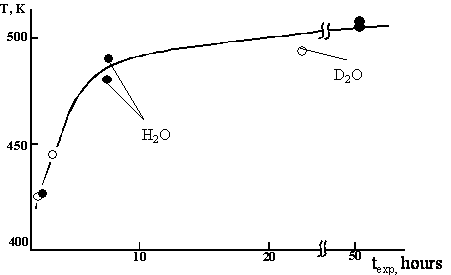
Fig.3
These results could be easily
interpreted if we assume that, like in the case of compression, plastic deformation
of metal during cutting is accompanied by hydrogen transport into bulk of
metal. Hydrogen at first concentrated in the outer part of chips redistributes
with time by (a) diffusion into the bulk of material which leads to smoothing
of concentration and (b) hydrogen transport to surface, recombination and
evolving into the gas phase.
Redistribution of hydrogen in the bulk
along with lowering of its total content in the sample leads to the observed
high temperature peak shift in the TPD spectra and diminishing of integral
intensity with time.
Briefly the mechanism of hydrogen
transfer seems to be the following. During plastic deformation both in the case
of compression and cutting fresh metal surface is produced. The hydrogen
containing molecules of media like water, saturated or unsaturated hydrocarbons
react with this highly chemically active surface producing adsorbed hydrogen
atoms after partial dehydrogenation. We must also notice that adsorbed hydrogen
atom has to overcome high activation barrier to penetrate into bulk, the
transfer being endothermic. Thus the rate of hydrogen permeation is noticeable
only at high temperature and considerable hydrogen concentration (pressure).
Temperature of chips in the moment of shearing usually does not exceed 600-700
C. The rates of diffusion as well as hydrogen concentration in the near surface
region are too small for hydrogen to penetrate deep enough into the outermost
layers of metal.
The depth of permeation (R) could be
estimated by formulae
R2=Dt
where D - coefficient of diffusion, t= l/v, v - cutting speed, 1 and t, respectively,
typical shearing zone and contact time, during which plastic deformation occur.
Usually l = 1mm, v = 0.1m/sec, then contact time is of the order of 10-2
sec, and for relevant diffusion coefficient the depth of hydrogen permeation,
even overestimated, could not exceed 10-3 cm. However, as hydrogen
permeation does take place, it is worth assuming that high rate of hydrogen
transport into the bulk is caused by very high instantaneous "heating"
of the crystal lattice freedom degrees responsible for hydrogen transfer.
Cutting in ethanol
TPD spectrum after cutting in ethanol
is shown in Fig. 4. The position of peak maximum is noticeably shifted to
higher temperature compared with water. As concerning peak intensities, though
they are of' the same order, however, the total amount of hydrogen desorbed is
smaller for ethanol than for water. TPD spectrum of chips after cutting in
ethanol-water solution (1:1) in comparison with pure water is shown in Fig. 4.
Peak maximum for solution is in the intermediate position. We must point out
that cutting in organic media always leads to high temperature maximum shift in
comparison with water.

Fig.4
This fact can be explained by taking
into account special physical properties of water, which lead to higher race or
cooling or, in other words to hardening and concentrating of hydrogen only in
the thin outermost layer of the sample, while cutting in water. After cutting
in air (containing water vapor) the peak is positioned at higher temperature,
although hydrogen-containing compound is the same in both cases, cooling
conditions are different and hydrogen can diffuse deeper into bulk. Ethanol-water solution has an intermediate
thermocondactivity property, which lead to intermediate peak position.
Cutting in Vaseline
oil-water emulsion
Water emulsions or suspensions of
polymers are known to be among the most effective metal working fluids. A
hypothesis was suggested that hydrogen plays an important role in facilitation
of plastic deformation of the treated material. TPD experiment data for chips
after cutting in Vaseline oil – H2O (D2O) emulsion in
comparison with water is shown in Fig. 5. It is seen that for two observable
hydrogen peaks in the TPD spectrum of emulsion the low temperature peak is
represented by hydrogen absorbed mainly from water (consists of D2
for D2O emulsion) and the high temperature peak by hydrogen mainly
from organic compound (consists of H). The appearance of two additive peaks in
the TPD spectrum points out that there exist two different contact regions,
where cutting proceeds either in water or in hydrocarbon. But the most
interesting thing is that addition of an emulator leads to high increase in
intensity of the low temperature peak, i.e. promotes the permeation of hydrogen
from water into treated material. Maybe this fact is connected directly with
nigh efficiency of emulsions as cutting fluids in metalworking.
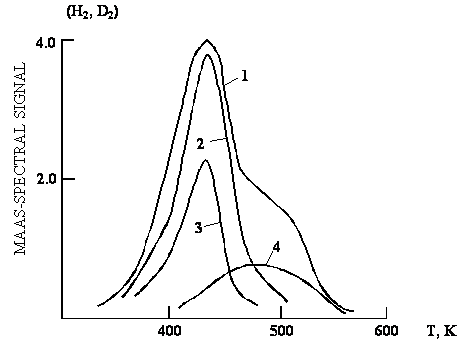
Fig.5
Conclusions
l. Plastic deformation during
compression as well as cutting of metal is followed by hydrogen transport into
bulk of metal.
2. After plastic deformation in water
hydrogen is concentrated in thin outermost layer while in the case of organic
media its spatial distribution is wider.
3. Hydrogen transport during plastic
deformation could not be described in terms of equilibrium thermodynamics of
diffusion process and some other theoretical approaches.
4. Adding small amounts of organic emulators
causes high increase of hydrogen transfer into metal, water being the main
source of this hydrogen.
List of used literature.
1. Ñîøêî À.È.,  ñá.: Ïîëèìåðû â òåõíîëîãè÷åñêèõ ïðîöåññàõ îáðàáîòêè ìåòàëëîâ. Êèåâ: Íàóê. Äóìêà, 1977, ñ. 7-15.
2. Øâåä Ì.Ì., Ñëàáêîâñêèé È.Ñ., Ñûíèêî Â.Ñ., Ôèçèêî-õèìè÷åñêàÿ ìåõàíèêà ìàòåðèàëîâ, 1970, ¹3, ñ.11-112.
3. Øìåëåâ Á.À. Â êí.: Ìåòîäû îïðåäåëåíèÿ è èññëåäîâàíèÿ ñîñòîÿíèÿ ãàçîâ â ìåòàëëàõ. Ì.: Íàóêà, 1968, ñ.45-48.
4. Morris M.A., Bowker M., King D.A., Chem.Kinetics, 1948, v.19, ð.1-179.
5. Êóäðÿâöåâ Â.Í., Âàëàêèí Þ.Ï., Ëÿõîâ Á.Ô., çàâîä. ëàá., 1968, 24, ¹2, ñ.196-199.
6. Ôàñò Äæ.Ä. Âçàèìîäåéñòâèå ìåòàëëîâ ñ ãàçàìè. Ïåð. ñ àíãë. Ì.: Ìåòàëëóðãèÿ, 1975, 352ñ.
7. Benziger J.B., Madix R.J., J. Catalysis, 74, ¹1, ð.55-66.
8. Ëîñåâ Â.Âë., ×åð÷åëü Î.Â., Êóïàíåíêî È.Â., Ýíòåëèñ Ñ.Ã., Êèíåòèêà è êàòàëèç, 1988, 29, ¹2, ñ.860-865.
9. Øâåä Ì.Ì. Èçìåíåíèå ýêñïëóàòàöèîííûõ ñâîéñòâ æåëåçà è ñòàëè ïîä âëèÿíèåì âîäîðîäà. Êèåâ: Íàóê. Äóìêà, 1985, 119 ñ.
10. Konig W., Vits R. Lubrication in metal woking, 3rd Int. Coll., 1982, v.2, ð.62.
11. Áîêøòåéí Á.Ñ. Äèôôóçèÿ â ìåòàëëàõ. Ì.: Ìåòàëëóðãèÿ, 1978, 247ñ.