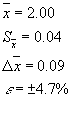Strusovskaya
O.G., Bebyakova N.A., Buyuklinskaya
O.V., Artemyeva A.P., Afonina S.A, Vidineev M.P
The Northern
State Medical University, Arkhangelsk, Russia
Determination of
acute toxicity of Cochlearia officinalis (Crucifereae
L.)
Lozhechnitsa or lozhechnaya (scorbutic) grass Cochlearia officinalis, genus of plants
of the Cruciferous family (Cruciferae L.) is a biennial (dicyclic)
plant. Cochlearia officinalis widely grows on the islands of the Solovetsky
archipelago. Due to a wide range of pharmacological activity it is used in traditional medicine. It
has anti-inflammatory, diuretic, antispasmodic and other effects. Content of ascorbic acid in the
grass is close to that in hips. It
is established that the chemical composition of Cochlearia officinalis includes polyhydroxylated nortropane alkaloids
[1], that creates the prerequisite for the plant study as a hypoglycemic agent. On the other hand, the presence of
alkaloids requires the determination of the plant toxicity.
During carrying out toxicological studies the nature
and severity of the damaging effect of a substance on the body are assessed. Studies are
conducted on small laboratory animals.
The first step of a toxicological study is to determine "acute"
toxicity at single dosing or intermittent administration of the substance over short
intervals (every 3-6 hours) during the day. Determination of acute toxicity allows
to establish tolerable, toxic and lethal doses, identifyeng the causes of animal
deaths [2].
The aim of the study was to determine acute
toxicity of Cochlearia officinalis
infusion. The object of the study was the air-dried aerial parts of Cochlearia officinalis, harvested during
the flowering period (July 2010) on two islands of the Solovetsky archipelago
(Great Muksalma and Anzer). The Cochlearia
officinalis infusion was prepared in accordance with the requirements of
the Pharmacopoeia of the USSR XI ed. [3]. For this purpose, the air-dry raw material of Cochlearia officinalis was crushed and
sieved through laboratory sieves S30/50 (State Standart 3826-82) with aperture sizes
0.5 mm and 3.0 mm. Purified water of room temperature was added to the
exact mass, about 1.0 g, of the crushed plant material with the particle size
not exceeding 3.0 mm (Pharmacopoeia’s article 42-2619-97) in a ratio of 1:10 (g
/ ml) and the mass was infused at frequent stirring on a boiling water
bath for 15 min. Then the infusion was cooled at room temperature for one hour,
with the plant material being pressed in a perforated glass. Based on the data collected
the water
absorption factor values were calculated (Table 1).
Table 1
Results of determination of water
absorption factor of
the Cochlearia officinalis air-dry raw material
|
mass of
air-dry raw material, g * |
amount of
purified water, ml |
amount of
the infusion, ml |
water
absorption factor |
metrological
characteristics |
|
1,02530 |
10,0 |
9,88 |
2,0 |
|
|
1,01265 |
10,0 |
9,81 |
1,9 |
|
|
0,99985 |
10,0 |
9,79 |
2,1 |
|
|
0,99790 |
10,0 |
9,88 |
2,0 |
|
|
1,01295 |
10,0 |
9,81 |
1,9 |
|
|
1,01660 |
10,0 |
9,79 |
2,1 |
Calculations
are made for the raw materials with humidity 8.9%
The infusion for determination of acute toxicity was prepared in a
similar way by adding purified water to 10 g of powdered plant material, taking
into account the calculated water absorption factor of 120 ml. For the
toxicological study healthy adult rats (both males and females) of the
"Vistar" type, weighing 180 – 220 g, having been quarantined for 14
days, were used. Keeping
of experimental animals met the working sanitary regulations on arrangement, equipment
and maintenance of experimental biological clinics (vivariums) [4]. The animals were kept with constant
access to water, on a standard diet, feeding was carried out at a fixed time.
The experiment was conducted in accordance with the
guidelines on the preclinical study of new pharmacological agents [2]. During
the study of toxic doses of the Cochlearia
officinalis infusion there were four experimental and control groups with 6
animals in each group. Every day in the morning the animals were injected
intraperitoneally with the help of polyurethane tube with 2 ml of the Cochlearia officinalis infusion; intact
control animals were injected with the same volume of 0.9% sodium chloride
solution, and then the general state and behavior of the animals were monitored. Administration
of the infusion was repeated every three hours during the day. During the
experiment the animals were not fed but had free access to water. During the
first day of the experiment the animals were under constant supervision. The
observation lasted for two weeks. The following parameters were regularly
recorded: general state, behavior characteristics, intensity and nature of
motor activity, presence/absence of seizures, response to tactile, auditory and
visual stimuli, condition of hair and skin, color of mucous membranes, amount
and consistency of stool, frequency of urination and coloringurine, food and
water consumption, weight change.
The strategy of the animal behavior was assessed using the "open
field" installation. It is used to study the behavior of small rodents and
allows to estimate the level of emotional behavioral reactivity of animals [5].
The "open field" installation is a square arena with dimensions 100 × 100 cm, divided into 25 (5 cm × 5 cm) equal squares
with the side 20 cm with a hole (2.5 cm in diameter) each, surrounded by the
wooden barrier with height of 40 cm and opened from the top.
Behavioral responses of the animals were recorded
every 3 hours during the first day of the experiment for nine hours. During 3
min the following parameters were monitored: the horizontal locomotors activity
(number of squares crossed), exploratory behavior (number of whole peeping),
time of grooming and time of passive sitting. The data
were presented as ratios to the total time spent in the "open field"
installation.The data on the behavioral
reactions of the animals obtained during the study are presented in Tab. 2. At
analyzing the strategies of the males’ behavior there were not identified any behavioral
changes for all investigated parameters during the whole experiment. In the
female group due to the infusion administration there was an increase of time of
passive sitting and reduction of horizontal locomotors activity. At the
same time the rates of exploratory behavior and grooming did not change during
the experiment. Reduced locomotors activity of the females could be related
to both the drug effect and the features of the females’ adaptive strategies. Attention
is drawn to the fact that the horizontal motor activity of the females
decreased due to the infusion administration compared with the initial
activity, but then during the whole experiment there was no progressive
reduction of
motor activity.
Table 2
Strategy of the animal behavior in the "open field" installation
|
Parameters |
Females |
Males |
||||||
|
Initial state |
After the infusion administration |
Initial state |
After the infusion administration |
|||||
|
3:00 |
6:00 |
9:00 |
3:00 |
6:00 |
9:00 |
|||
|
Time of
passive sitting (%) |
44,4 ±3,79 |
47,3±13,5 |
58,2±10,1 |
63,33±9,39 |
34,8±9,6 |
38,8±11,04 |
33,7±4,79 |
36,7±9,6 |
|
Horizontal
locomotor activity (sec) |
9,0±2,2 |
9,2±4,5 |
5,8±0,8 |
4,6±0,7 |
18,0±3,5 |
15,6±3,8 |
12,4±3,9 |
18,2±3,4 |
|
Exploratory
behavior (sec) |
5,8±0,7 |
6,4±1,8 |
3,2±1,4 |
2,8±1,2 |
5,0±1,7 |
7,8±1,9 |
6,4±2,1 |
3,8±1,2 |
|
Grooming
time (sec) |
2,0±0,8 |
1,2±0,2 |
1,4±0,6 |
1,0±0,45 |
1,0±0,7 |
1,6±1,1 |
2,4±0,8 |
2,0±0,4 |
During the 14 days monitoring of the experimental animals there were no changes
in the state of hair and skin, in comparison with the initial state, and with the
intact animals state. Also,
throughout the monitoring period, there were no changes in motor coordination,
food and water consumption, consistency and volume of fecal and urinary
frequency. There
were no significant changes in the animals’ body weight.
Thus, our data show no marked influence of the infusion on the
behavioral reactions of the animals, during the experiment there were no deaths,
pathological changes in external characteristics. Thereby the Cochlearia officinalis infusion can be referred
to the fourth hazard class (low hazard substances) [6].
References:
1. Brock A., Herzfeld T., Paschke R., Koch M., Dräger
B. Brassicaceae contain nortropane alkaloids // J.
Phytochem .- 2006 .- Vol. 67 .- Iss. 18 .- P. 2050-2057.
2. Guide to experimental (preclinical) study of new
pharmacological agents /R.U Habriev, O.L.Verstakova, E.V. Arzamastsev, E.A.
Babaian, et al // Edited by Corresponding Member.of Russian Academy of Medical Sciences, prof. R.U. Habrieva. - 2nd ed. Rev. and add. - Moscow. - "Publisher" Medicine. " - 2005. - 832 pgs.
3. State Pharmacopoeia USSR. / Eleventh ed., Vol. 2. Common
methods of analysis. Medicinal plant material
//Moscow.:Medicine.-1990 .- P. 147-148.
4. Sanitary
regulations on arrangement, equipment and maintenance of
experimental
biological clinics (vivariums).N1045-73.
5. Ayrapetyants M.G. Correction of
behavioral and physiological indicators of neurosis-like states of white rats
by administration of succinic acid / M.G. Ayrapetyants, I.P. Levshina, LV Nozdracheva, N. Shuikin // Zhurn. HNA. -
2001. - V. 51, ¹ 3. - S. 360-367.
6. GOST 12.1.007-76. Labour safety standards system. Harmful substances. Classification and general safety requirements dated
01.01.1977.