Gordina N.E.,
Teplyakova N.M., Teplyakova A.N., Prokof’ev V.Yu.
Ivanovo State
University of Chemical and Technology, Russia
Preparation of Granulated
LTA Zeolites from Mechanically Activated Mixtures of Metakaolin
Widely used method for the production of the zeolites is the
synthesis from metakaolin, where the Si:Al = 1:1 ratio corresponds to the
silicate modulus of LTA zeolite. Hydrothermal crystallization in alkaline
solution determines the future course of the process. The solutions of sodium
hydroxide and sodium aluminate are used for the crystallization. The process is
carried out in several stages with different concentrations of NaOH. The phase
composition is determined by both the concentration and temperature during the
crystallization process. The disadvantages of these two methods are high
sensitivity to reagent concentrations and temperature, a substantial duration
of the process as well as the formation of a large amount of waste waters.
We have shown [1, 2] that it is possible to realize the mechanochemical
synthesis (MCS) of zeolites in the activator mills. The advantage of MCS is the
use of dry mixtures that allows one to minimize the amount of liquid phase in
whole synthesis process. The goal of this work
was the investigation of the LTA zeolite synthesis process in the dry mixture
using kaolin raw material in the roller-ring vibratory mill. In order to
increase the content of the LTA zeolite crystalline phase, it is necessary to
optimize the stage of thermal treatment as well as to study deeper the stage of
hydrothermal crystallization.
It was shown that the MCS of LTA zeolite requires the
anhydrous ingredients (metakaolin, alumina, and sodium aluminate). The presence
of structural water in the raw materials (kaolin, aluminum hydroxide, and sodium
gidroalyuminat) gives the formation of undesirable sodalite or nepheline [2].
It was concluded that the synthesis of the LTA zeolite
requires the presence of sodium aluminate of cubic or tetragonal structures
with the lattice parameters close to those of the zeolite. These aluminates act
as a structure directing agent [1]. Sodium
aluminates with other crystal structures and with other lattice parameters
result in the formation of sodalite.
There is an optimal time of MCS, which is determined
by the synthesis of sodium aluminate with a required crystal structure. An
optimum time of the MCS in the vibratory mill was found. This time is
determined by two factors such as the synthesis of sodium aluminate with cubic
or thetragonal syngonis and the absence of sodium aluminate with other crystal
structures (for example, orthorombical syngony). For the vibratory roll-ring
mill used in this work, an optimal time of MCS is in the range of 5–7 min.
It was shown that the thermal treatment of the mixture
of metakaolin, sodium hydroxide and aluminum oxide which was preliminary
subjected to both mechanical activation in a vibration mill and granulation
results in the formation of the LTA zeolite. The optimum temperature of 600ºC
was determined. The amount of the zeolite was about 65 wt%, while the crystals
have the cubic shapes with the size of 0.2–0.3 μm and were combined into
the agglomerates with the sizes of up to 1 μm (Fig. 1). An increase in
process temperature leads to a decrease in the zeolite amount because the nepheline
is formed. The LTA zeolite crystals consist of multiple microblocks with a
sizes of about 1 μm and have the minimum level of crystal lattice
defects of about 0.15 %. It has been suggested that, during the thermal
treatment, the process of nucleation of a new phase is the limiting stage [3].


Fig. 1 SEM images of mixture
for zeolite synthesis after 5 min MCS and thermal treatment at 600°C (left) and 700ºC (right)
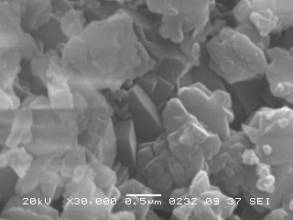

Fig. 2 SEM images
of mixture for zeolite synthesis after 5 min MCA and thermal treatment at 600 °C and hydrothermal crystallization at 2 (left) and 8 (right) mol/l
It was found that the hydrothermal crystallization in
aqueous NaOH solution allows one to obtain the amount of the LTA zeolite more
than 90 wt%. For the 2 mol/l NaOH solution, the crystals have an average size
of 0.6–0.8 μm and are combined into the aggregates with the sizes from 5 to
20 μm (Fig. 2). It was observed that the crystal lattice of the LTA zeolite
has a minimum defectiveness level of about 0.07 %. It is also shown that an
increase in the NaOH concentration up to 4 mol/l results in the formation of
the sodalite, while the size of the LTA zeolite particles becomes to be less
than 0.5 μm (Fig. 2). At 8 mol/l NaOH, only the sodalite phase is formed.
It was proposed that, at low NaOH concentrations, an increase in the amount of
the LTA zeolite is due to the growth of existing crystals. The high level of Na+
cations at higher NaOH concentrations leads to the destruction of the Double-4-Rings
and to linking of the sodalite cages through the Simple-4-Rings.
Reference:
1. Prokof’ev V.Yu. at al. // J. Mater. Sci.
2012. 47 (14). pp. 5385-5392. DOI: 10.1007/s10853-012-6421-3
2. Prokof’ev V.Yu. at
al. // Rus. J. Appl. Chem. 2012. 85 (7). pp. 1077-1082. DOI:
10.1134/S1070427212070142
3. Prokof’ev V.Yu. at al. // J. Mater. Sci. 2013. 48 (18). pp. 6276-6285. DOI: 10.1007/s10853-013-7425-3