Biochemical and
ultrastructural changes in mouse hepatocytes after the administration
of exogenous melatonin.
T. Król, M. Łysek-Gładysińska, H. A. Wieczorek,
K. Staszczyk, W. Trybus, A. Król, E. Trybus, A. Kopacz-Bednarska.
Department
of Cell Biology, Institute of Biology, Świętokrzyska Academy,
Świętokrzyska 15, 25-406 Kielce, Poland.
Abstract
The experiment was performed on male mice of the
Porton breed, aged 12 and 44 weeks. The animals were kept in standard
conditions, with a constant access to water and feed (with a 16% protein
content). In each age group, a control and an experimental group was chosen.
Mice belonging to the experimental group were given, through a feeding probe,
0.5 mg/kg b.w of melatonin for 14 days. The control animals received a solution
of 0.9% physiological salt through an analogical method.
Melatonin caused an
increase in the activity of the analyzed lysosomal enzymes in 44-week old males
and a decrease in the enzyme activity of 12-week old mice. These changes
correlated with the morphological profile changes in the studied hepatocytes.
Exogenous melatonin
administered for 14 days caused significant changes in the morphological
profile of the cell and an increase in the activity of the studied lysosomal
enzymes only in older specimens. It can therefore be concluded that melatonin
stimulated the degradative processes only in 44-week old mice.
Key
words: Melatonin, cathepsin D and L, acid phosphatase, β-glucuronidase,
mouse, liver
Introduction
Much attention has lately been given to
melatonin, because, as numerous papers suggest, it can positively influence the
life-length and improve its “quality” [42,41]. It was discovered by Lerner in
1959 [12,31,59]. Melatonin (N-acetylo-5-metoksytryptamine)
is formed in a four-step process from the amino acid precursor L-tryptophan
. The key enzyme in the biosynthesis of melatonine is serotonine
N-acetyltransferase [18,61]. Its biosynthesis takes place mainly in the pineal
gland and can also happen in the retina and, to a point, in the Harder gland and the intestines [3].
Melatonin synthesized in the pineal
gland is released into the bloodstream and the cerebrospinal fluid and through
there reaches the tissues of the body, where it exerts its physiological
effects [53,54].
Certain premises exist which suggest a
connection between melatonin and the aging process. From the works of, among
others, Hause [13,39], it seems that the rate of melatonin production isn’t
constant and decreases with age (lowering slowly until the age of 40 to 50 and
then much faster).
The available literature discusses the
role of melatonin in delaying the aging process and the prevention of neoplasm
therapy [40,51]. Melatonin is
attributed, among its other effects, to remove damage arising as a result of
the aging process [44,48].
However, many unexplained mechanisms of
the action of melatonin still remain. Very little data can be found on the
effect of melatonin on the lysosomal compartment, which is the first to react
in situations of disturbed cell homeostasis [2,8,10,26,27,24,25,63,62,62]
Materials and Methods
The animals used in the experiment were 40 male mice of the Proton
breed, aged 12 and 44 weeks. The animals were kept in a room with a naturally
regulated light-to-dark ratio, LD 12:12, were feed with dry granulated Murigram
feed with 16% protein content and had a constant access to water. In each age
group the animals were divided into a control group and an experimental group.
The experimental animals were given, through a feeding probe, daily
for 14 days, at a set time (900), melatonin in the dosage of
0.5mg/kg b.w. The control animals were given a solution of 0.9% physiological salt in an analogical
way.
After 14 days, the animals of all groups were decapitated, following
which slices of liver were taken for biochemical studies. The liver tissue was
homogenized in the temperature of +4 oC in a 0.25 M solution of
sucrose (1 g tissue: 7 ml sucrose). The homogenate was then centrifuged
differentially according to the method of Marzella and Glaumann [33].
In the obtained lysosomal fraction, the activity of select model
lysosomal enzymes was marked: cathepsine D and L (Cath. D and L, EC 3.4.23.5.,
EC 3.4.22.15.) according to the method of LANGNER et al. [28]; acid phosphatase
(AcP, EC 3.1.3.2.) according to the method of HOLLANDER [14];
β-D-glucuronidase (BGRD, EC 3.2.1.31.) by the method of BARRETT [4]. The
total protein content was marked by a modified Lowry method [21]. The activity
of the studied enzymes was expresses in μmol/mg of protein/hour. The
results obtained were expressed as means
and standard deviations.
Furthermore, fragments were taken for
microscopic studies. Initial fixation was performed in 3% glutaraldehyde. For further fixation,
2% osmium tetroxide was used. Contrasting was done in 2% uranyl acetate.
Dehydrated slices were sealed in Epon 812 (Serva, Germany). Additional
contrasting of ultra-thin slices was done in uranyl acetate and lead citrate
according to the method of Marzella and Glaumann [34]. The photographs were
taken with the use of the TESLA BS 500 electron microscope.
The experiment was conducted according to the recommendations of the
Ethical Committee for Animal Experimentation.
Results
The obtained results are shown in
figures 1-2 and photographs 1-4. They present the changes in the activity of
observed lysosomal enzymes in the liver of male 12- and 44-week old mice,
caused by the administration of melatonin.
14-day
action of melatonin in the dose of 0.5 mg/kg b.w. ( fig 1) caused, in 12 week
old males, a statistically significant decrease in the activity of cathepsin D
and L (to 71%, p<0,001) and acid phosphatase (to 57%, p<0,001). No
statistically significant changes were observed in the case of β
–D-glucuronidase (108%). The administered dose of melatonin did not reveal
significant changes in the ultrastructure of the hepatocytes observed (phot. 2)
in comparison to the control cell (phot. 1).
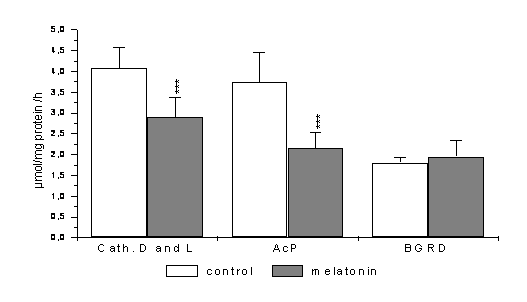
Fig. 1 Changes in the activity of lysosomal enzymes in mouse liver of
12-week old mice after 14 days of melatonin administration in the dose of 0,5
mg/kg b.w.
*** p< 0,001- statistically significant
differences
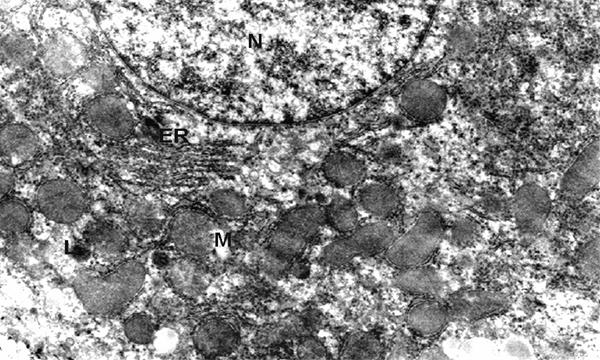
Phot. 1.
Electron micrographs of hepatocyte fragment of control group - 12 weeks old.
(N) nucleus, (ER) endoplasmic reticulum, (M) mitochondria, (L) lysosomes, x 8.500
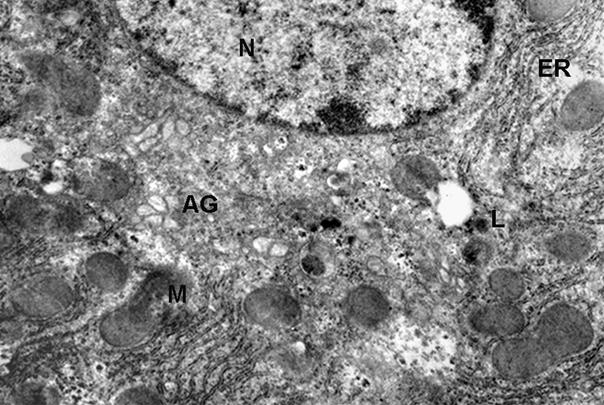
Phot. 2.
Electron micrographs of hepatocyte fragment of mouse - 12 weeks old after
administrating of melatonine in the dose of 0.5 mg/kg b.w. (N) nucleus, (ER)
endoplasmic reticulum, (M) mitochondria, (L) lysosomes, (AG) Golgi apparatus, x
13.500
The consequence of administering melatonin to 44 week old males (fig. 2)
was a highly statistically significant increase of the activity, of cathepsin D and L (to 199%, p<0,001), acid
phosphatase (to 210%, p<0,001) and β-glucuronidase (to 262%,
p<0,001).
The observation of the morphological profile of the cell revealed a well
developed rough endoplasmatic reticulum and significantly enlarged mitochondria
(phot. 4) in comparison to the control cell (phot. 3).
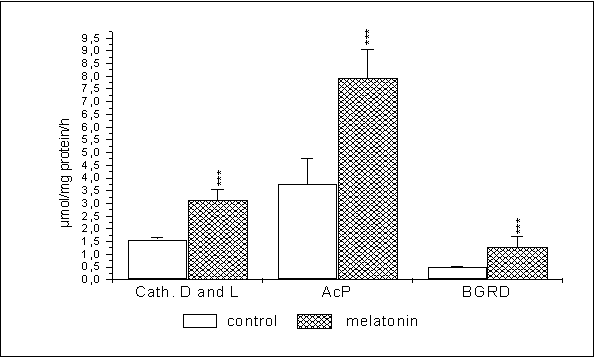
Fig. 2. Changes in the
activity of lysosomal enzymes in mouse liver of 44-week old mice after 14 days
of melatonin administration in the dose of 0,5 mg/kg b.w.
*** p< 0,001- statistically significant differences
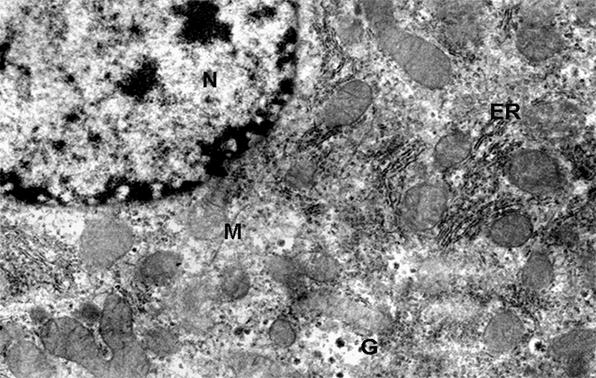
Phot. 3. Electron
micrographs of hepatocyte fragment of control group - 44 weeks old. (N)
nucleus, (ER) endoplasmic reticulum, (M) mitochondria, (G) glycogen granule, x 13.000
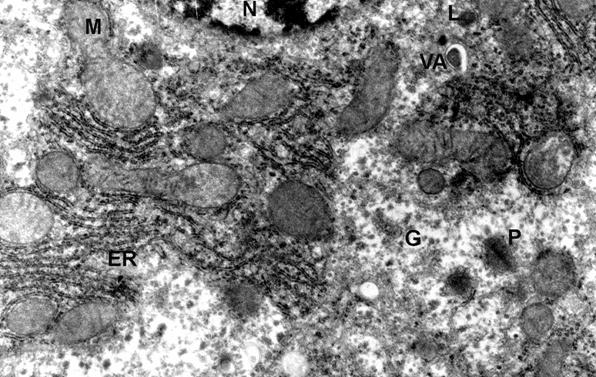
Phot. 4. Electron micrographs of hepatocyte
fragment of mouse - 44 weeks old after administrating of melatonine in the dose
of 0.5 mg/kg b.w. (N) nucleus, (ER) endoplasmic reticulum, (M) mitochondria,
(L) lysosomes, (G) glycogen granule, (VA) vacuole autophagic, (P) peroxisome, x
12.500
Discussion
Numerous papers show that melatonin can strengthen the immunological
system, which gets weaker with age [54]. Increasing, among others, the
production of lymphocytes T, which enable resistance to certain diseases,
melatonin softens the side effects of chemotherapy and regulates the natural
sleeping rhythm. The effectiveness of this hormone in treating sleeping
disorders, resulting from, for example, a quick change of time zones or from
old age, has been shown [5,9,13,20,29,30,46,47,55,58].
It has also been shown, that this
hormone lowers the content of lipid peroxidation products, the increase of
which accompanies certain diseases, such as the Parkinson disease or the
Alzheimer disease [36,63]. The observations, that with age the
concentration of endogenous melatonin lowers, in the result of which the
activity of free radicals increases and so does the susceptibility to different
diseases, are especially interesting [19,37,38,39,42,47, 45,52,56].
This hormone displays good
dissolubility, which allows it to freely enter the interior of all cells, and
its action is not limited to only cellular membranes [6,11]. The highest
concentration of melatonin can be found in the nucleus, where it is
specifically bound by nucleoproteins and takes part in protecting DNA from the
chemical effect of carcinogens and acts in delaying the aging process [1].
The gradual decrease in melatonin
secretion which accompanies aging may disturb membrane transport, as well as
the cellular and organism metabolism, leading to a disturbance in the endogenous
homeostasis and contributing to the arising of diseases associated with old age
[32,35,50].
The analysis of the activity of the enzymes observed in 12-week old mice
after 14 days of exogenous melatonin administration showed a statistically significant
decrease in the activity of studied lysosomal hydrolases (fig.1). However, the
morphological studies did not show any significant changes in the
ultrastructure of the cell encumbered with melatonin, in comparison to the
morphological profile of the control cell (phot.1,2). The above-mentioned
results show a decrease in the degradative processes in the liver cells studied
( fig.1).
From the works of Parmar et al. [37]
and Pawlikowski et al. [40] it can be concluded that taking prophylactic
melatonin at an appropriate age can decrease the hormonal disturbances
appearing as a result of a low level of this hormone in the body, especially
during menopause or andropause.
Our data too show that melatonin caused, in the liver of aging (44-week
old) male mice, biochemical changes expressing in a statistically significant
increase in the activity of Cath D and L, AcP and BGRD (fig.2). Ultrastructural
studies show an increase in the number of mitochondria of a slightly swelled
structure (phot. 4). These changes are probably an expression of the cellular
adaptation to the increasing energy need. The increased number of glycogen
granules might also suggest an intensified operation of the cell. These changes
correlate with the biochemical changes, which suggest a stimulation in
degradative processes in 44-week old specimens.
When comparing the observed enzymatic reactivity of 44-week old males in
comparison to 12-week old males, it was concluded that 14 days of melatonin
administration caused a statistically significant increase in most of the
studied hydrolases only in 44-week old males and not in the younger, 12-week
old males.
The above-mentioned data suggest that exogenous melatonin stimulated
degradative processes only in aging, 44-week old males. Numerous papers show
[8,22,23,57] that the aging process is accompanied by changes in the lysosomal
system, indicating, among others, an inhibition of the degradative processes
within the cell. The results obtained (fig.2, phot. 3, 4) allow us to suspect
that melatonin, by supporting the degradative process in aging (44-week old)
specimens, prevents the intracellular gathering of damaged proteins, which are
the source of many diseases associated with old age.
These results are a confirmation of numerous other works [7,17]. Other
authors [16,49,60] suggest that melatonin, by, among other factors, its
antioxidant properties, plays a significant part in slowing the aging process.
In conclusion, it can be said that exogenous melatonin caused specific,
age-dependant biochemical and ultrastructural changes, which were an expression
of a precisely defined cellular function.
Melatonin, by influencing the overall processes taking place in the
cell, contributed to the restoration of degradative processes in aging mice. It
can therefore be suggested, that the supplementation of the melatonin
deficiencies, which increase with age, may be a chance for preventing diseases
in aging specimens, as well as for delaying the aging processes.
References
[1] Acuna-Castroviejo, D., Reiter, R.J., Menendez-Pelaez,
A., Pablos, M.I., and Burgos, A. 1994, Characterization of
high-affinity melatonin binding sites in purified cell nuclei of rat liver,
Journal of Pineal Research, 16, 100-112.
[2] Arai, K., and Ohkuma,
S. 1995, Metabolic pathway of the degradation of
macromolecules by lysosomal enzymes, Japanese Journal of Clinical Medicine, 53,
2904-2910.
[3] Arendt, J. 1998,
Melatonin and the pineal gland: influence on mammalian seasonal and circadian
physiology, Reviews of Reproduction, 3, 13-22.
[4] Barrett, A.J. 1972,
Lysosomal enzymes. In ,,Lysosomes’’, A Laboratory Handbook. J.T. Dingle [ed]
North Holland, Publ. Co Amsterdam, 46-135.
[5] Baskett, J.J., Broad, J.B., Wood, P.C., Duncan,
J.R., Pledger, M.J., English, J., and Arendt, J. 2003, Does
melatonin improve sleep in older people? A randomised crossover trial, Age Ageing, 32, 164-170.
[6] Costa, E.J., Lopes, R.H., and Lamy-Freund, M.T.
1995, Permeability of pure lipid bilayers to melatonin, Journal of Pineal
Research, 19, 123-126.
[7] Cuervo, A.M., and Dice, J.F. 1998a,
How do intracellular proteolytic systems change with age? Frontiers in
Bioscience, 3, 25-43.
[8] Cuervo, A.M., and Dice, J.F.
2000, When lysosomes get old, Experimental Gerontology, 35, 119-131.
[9] Czeisler, C.A., Shanahan, T.L., Klerman, E.B.,
Martens, H., Brotman, D.J., Emens, J.S,
Klein, T., and Rizzo, J.F. 1995, Suppression of melatonin
secretion in some blind patients by exposure to bright light, The New England
Journal of Medicine, 332, 6-11.
[10] Dunn, W.A., Jr 1994, Autophagy and
related mechanism of lysosome-mediated protein degradation, Trends Cell
Biology, 4, 139-143.
[11] Escames, G., Leon, J., Macias, M., Khaldy, H.,
and Acuna-Castroviejo, D. 2003, Melatonin counteracts
lipopolysaccharide-induced expression and activity of mitochondrial nitric
oxide synthase in rats, FASEB Journal, 17, 932-934.
[12] Fourtillan, J.B., Brisson, A.M., Fourtillan,
M., Ingrand, I., Decourt, J.P., and Girault, J. 2001,
Melatonin secretion occurs at a constant rate in both young and older men and
women, American Journal of Physiology and Endocrinol Metabolism, 280, 11-22.
[13] Haus, E., Nicolau, G.Y., Ghinea, E., Dumitriu,
L., Petrescu, E., and Sackett-Lundeen, L. 1996,
Stimulation of the secretion of dehydroepiandrosterone by melatonin in mouse
adrenals in vitro, Life Sciences, 58, 263-267.
[14] Hollander, V.P. 1970, Acid
phosphatases In ,,The enzymes’’, [ed]. Boyer P.D., Academic Press. London. 4,
449-498.
[15] Huether, G. 1996,
Melatonin as an antiaging drug: between facts and fantasy, Gerontology, 42,
87-96.
[16] Inserra, P., Zhang, Z., Ardestani, S.K.,
Araghi-Niknam, M., Liang, B., Jiang, S., Shaw, D., Molitor, M., Elliott, K.,
and Watson, R.R. 1998, Modulation of cytokine production by
dehydroepiandrosterone (DHEA) plus melatonin (MLT) supplementation of old mice,
Proceeding of the Society for Experimental Biology and Medicine, 218, 76-82.
[17]
Jurgowiak, M.R., and Oliński, R. 1995, Wolne rodniki a starzenie się, Kosmos,
44, 71-88.
[18] Karasek, M., and Reiter, R.J. 1997, Czy szyszynka odgrywa rolę w
procesie starzenia? Polish Journal of Endocrinology, 48, 277-284.
[19] Karasek, M. 1998, The pineal gland, melatonin and
aging, Polish Journal of Endorinology, 2, 33-40.
[20] Kendler, B.S. 1997, Melatonin;
media hype or therapeutic breakthrough? Nurse Practitioner, 22, 66-67,
71-72, 77.
[21] Kirschke,
H., and Wiederanders, B. 1984, Methoden zur Aktivitätsbestimmung von
Proteinases, Martin-Luther-Universität Halle-Wittenberg, Wissenschaftliche
Beitrage Halle/Salle, 11-17.
[22] Klionsky, D.J., and Emr, S.D. 2000, Autophagy as
a regulated pathway of cellular degradation, Science, 290, 1717-1721.
[23] Kovacs, A.L., Rez, G., Palfia, Z., and Kovacs,
J. 2000, Autophagy in the epithelial cells of murine seminal vesicle in
vitro. Formation of large sheets of nascent isolation membranes sequestration
of the nucleus and inhibition by wortmannin and 3-ethyladenine, Cell and Tissue
Research, 302, 253-261.
[24] Król, T., and Kołątaj, A. 2000,
Activity of the lysosomal system in mouse liver after hydrocortisone
administration, Acta Biologica Cracoviensia Series Zoologia, 42, 29-32.
[25] Król, T., Witek, B., Wieczorek, A., and
Łysek-Gładysińska, M. 2002, Effect of prolonged
starvation on autophagy processes in mouse liver cell, Acta Biologica
Cracoviensia Series Zoologia, 44, 53-60.
[26] Król, T. 1998a,
Activity of lysosomal system in mouse liver after taxol administration, General
Pharmacology, 30, 239-243.
[27] Król, T. 1998b,
Influence of colchicine on autodegradation in mouse hepatocytes, Acta Biologica
Cracoviensia, 40, 31-39.
[28] Langner, J., Wakil, A., Zimmermann, M., Ansorge, S., Bohley, P.,
Kirschke, H., and Wiederanders, B. 1973, Aktivitätsbestimmung proteolytischer
enzyme mit azokasein als substrat, Acta Biologica Medicine, 31,
1-18.
[29] Lechner, O., Dietrich, H., Santos, A.O., Wiegers, G.J., Schwarz, S.,
Harbutz, M., Herold, M., and Wick, G. 2000, Altered
circadian rhythms of the stress hormone and melatonin response in lupus-prone
MRL/MP-fas(Ipr) mice, Journal of Autoimmunity, 14, 325-333.
[30] Leibenluft, E., Schmidt, P.J, Turner E.H, Danaceau, M.A., Ashman,
B.S., Wehr, T.A., and Rubinow, D.R. 1997, Effects of
leuprolide-induced hypogonadism and testosterone replacement on sleep,
melatonin, and prolactin secretion in men, The Journal of Clinical
Endocrinology and Metabolism, 82, 3203-3207.
[31] Lerner, A.B., Case, J.B., and Hinzelman, R.W. 1959, Structure
of melatonin, Journal of the American Chemical Society, 81, 6084-6085.
[32] Lunenfeld, B. 2002,
Replacement therapy in the aging male, Journal of Endocrinological
Investigation, 25, 2-9.
[33] Marzella
L., and Glaumann H. 1980a, Increased degradation in rat liver induced by
vinblastine I: Biochemical characterization, Laboratory Investigation, 42,
8-17.
[34] Marzella
L., and Glaumann H. 1980b, Increased degradation in rat liver induced by
vinblastine II: Morfological characterization, Laboratory Investigation, 42,
18-27.
[35]
Murray, R.K., Granner, D.K., Mayes, P.A., and Rodwell, V.W. 1995, Biochemia Harpera,
Państwowy Zakład Wydawnictw Lekarskich, Warszawa, 585-644.
[36] Pappolla, M.A., Sos, M., Omar, R.A., Bick,
R.J., Hickson-Bick, D.L., Reiter, R.J., Efthimiopoulos, S., and Robakis, N.K.
1997, Melatonin prevents death of nuroblastoma cells exposed of Alzheimer
amyloid peptide, Journal of Neurosciences, 17, 1683-1690.
[37] Parmar, P., Limson, J., Nyokong, T., and Daya,
S. 2002, Melatonin protects against copper-mediated free radical damage,
Journal of Pineal Research, 32, 237-242.
[38]
Pawlicki, B.
1996, Szyszynka - Trzecie oko boga Sziwy, Wszechświat, 97, 89-92.
[39]
Pawlicki B. 1999,
Rola szyszynki w fizjologii i patologii, Kosmos, 48, 29-42.
[40] Pawlikowski, M., Kolomecka, M., Wojtczak, A.,
and Karasek, M. 2002, Effect of six months melatonin treatment
on sleep quality and serum concentrations of estradiol, cortisol,
dehydroepiandrosterone sulfate, and somatomedin C in elderly women,
Neuroendocrinology Letters, 23, 17-19.
[41] Pierpaoli, W., and Maestroni, G.J.
1987, Melatonin: a principal neuroimmunoregulatory and anti-stress hormone: its
anti-aging effects, Immunology Letters, 16, 355-361.
[42] Pierpaoli, W., Regelson, W., and Colman, C. 1995,
Cud melatoniny, Wydawnictwo AMBER, 17-106.
[43] Qian, SZ., Cheng, Xu., Y., and Zhang, J.
2000, Hormonal deficiency in elderly males, International Journal of Andrology,
23, 1-3.
[44] Regelson, W., and Colman C.: 1997.
Obietnica superhormonów, Wydawnictwo Amber, 38-156.
[45] Reiter, R., Tang, L., Garcia, J.J., and Munoz-Hoyos, A. 1997, Pharmacological
actions of melatonin in oxygen radical pathophysiology, Life Sciences, 60,
2255-2271.
[46] Reiter, R.J. 1993, The melatonin
rhythm: both and clock and a calendar, Experientia, 49, 654-664.
[47] Reiter, R.J. 1998, Oxidative damage in the
central nervous system: protection by melatonin, Progress in Neurobiology, 56,
359-384.
[48] Rohr, U.D., and Herold, J.
2002, Melatonin deficiencies in women, Maturitas, 41, 85-104.
[49] San Martin, M., and Touitou, Y. 2000,
DHEA-sulfate causes a phase-dependent increase in melatonin secretion : a study
of perifused rat pineal-glands, Steroids, 65, 491-496.
[50] Schulman, C., and Lunenfeld, B. 2002, The
ageing male, World Journal of Urology, 20, 4-10.
[51] Sener, G., Tosun, O., Sehirli, A.O., Kacmaz,
A., Arbak, S., Ersoy, Y., Ayanoglu-Dulger, G. 2003,
Melatonin and N-acetylcysteine have beneficial effects during hepatic ischemia
and reperfusion, Life Sciences, 72, 2707-2718.
[52]
Skrzydlewska, E., Sulkowska, M., Makieła, M. 2001, Zmiany
wewnątrzkomórkowej degradacji białek w procesie starzenia
się organizmu, Postępy Higieny i Medycyny Doświadczalnej, 55,
467-481.
[53]
Skwarło-Sońta, K. 1994, Immunomodulacyjne działanie melatoniny,
Neuroimmunologia, 45-58.
[54] Skwarło-Sońta, K.
1996, Functional connections between the pineal gland and immune system, Acta
Neurobiologicae Experimentalis, 56, 341-357.
[55]
Słowińska-Klencka, D., and Lewiński, A. 1993, Rola melatoniny w fizjologii
człowieka. Znaczenie melatoniny w patogenezie chorób afektywnych i
zaburzeń chronobiologicznych. Melatonina a proces starzenia. Melatonina
a nowotwory, Postępy Higieny i Medycyny Doświadczalnej, 43, 267-276.
[56] Srinivasan, V. 2002,
Melatonin oxidative stress and neurodegenerative diseases, Indian Journal
Experimental Biology, 40, 668-679.
[57] Terman, A. 1995, The
effect of age on formation and elimination of autophagic vacuoles in mouse
hepatocytes, Gerontology, 41, 319-326.
[58] Tosini, G., and Fukuhara, C.
2003, Photic and circadian regulation of retinal melatonin in mammals, Journal
of Neuroendocrinology, 15, 364-369.
[59] Tricoire, H., Locatelli, A., Chemineau, P., and
Malpaux, B. 2002, Melatonin enters the cerebrospinal fluid
through the pineal recess, Endocrinology, 143, 84-90.
[60] Van Rensburg, S.J., Daniels, W.M., Van Zyl,
J.M., and Taljaard J.J. 2000, A comparative study of the
effects of cholesterol, beta-sitosterol, beta-sitosterol glucoside, dehydroepiandrosterone sulphate
and melatonin on in vitro lipid peroxidation, Metabolic Brain Disease, 15,
257-265.
[61] Vanecek, J. 1998,
Cellular mechanisms of melatonin action, Physiological Reviews, 78, 687-721.
[62]
Wójtowicz, Z., Stelmasiak, Z., Kis, G., and Obel, J.
1995, The activity of lysosomal enzymes in visual cortex of rabbits during
experimental diabetes, Folia Neuropathologica, 33, 159-162.
[63] Wolden-Hanson, T., Mitton, D.R., McCants, R.L.,
Yellon, S.M., Wilkinson, C.W., Matsumoto, A.M., and Rasmussen, D.D. 2000,
Daily melatonin administration to middle-aged male rats suppresses body weight,
intraabdominal adiposity, and plasma leptin and insulin independent of food
intake and total body fat, Endocrinology, 141, 487-497.