A.V.
Yudintsev1, V.M. Trusova1, G.P. Gorbenko1, T.
Deligeorgiev2,
A.
Vasilev2,
1V.N. Karazin Kharkov
National University, 4 Svobody Sq., Kharkov,
61077
2Department of
Applied Organic Chemistry, Faculty of Chemistry, University
of Sofia, Bulgaria
Lanthanide effect on structural state of model
membranes
Liposomes, spherical self-closed structures formed by one
or several concentric lipid bilayers currently represent a major interest for drug
delivery. While their lipidic bilayer
help solubilizing hydrophobic compounds, their
internal aqueous center provides a way of encapsulating hydrophilic drugs. Liposome-based
delivery systems are particularly attractive due to a number of advantages,
such as biocompatibility, complete biodegradability, low toxicity, ability to
carry both hydrophilic and lipophilic payloads and
protect them from chemical degradation and transformation, increased
therapeutic index of a drug, improved pharmacokinetic and pharmacodynamic
profiles compared to free drugs, reduced side effects, etc. Development of liposomal carriers is
heavily based on the evaluation of membrane-partitioning and bilayer-modifying
properties of the drug. This is important not only for achieving maximum
payload without compromising liposome stability, but also for prediction of
therapeutic and toxic effects of a certain compound, because membrane interactions
may prove critical for drug absorption, distribution, metabolism and
elimination in an organism. Of particular
importance is the development of liposomal formulations of new classes of antineoplastic drugs with alternative mode of cytotoxic action and nonoverlapping
mechanisms of drug resistance. One of such classes is represented by lanthanide
coordination complexes which have been reported to possess high cytotoxic potential.
In the present work we concentrated our efforts on the pre-formulation
studies of the two synthesized Eu(III) coordination complexes referred to here as LC1 and
LC2. More specifically,
our goal was twofold: i) to characterize membrane
partition properties of LC, and ii) to clarify the effects of these compounds
on physicochemical properties and structural state of the phosphatidylcholine
(PC) model lipid membranes.
a) b)
b)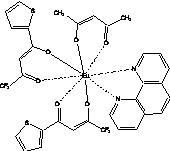
Fig. 1. Chemical structure of Eu(III) coordination complexes: a) LC1, b) LC2
LC1 and LC2 are
asymmetric Eu(III) coordination complexes with
diverse O-containing chelate ligands
which are thought to serve at least two main functions Ц bind tightly Eu(III), providing the
rigidity of the whole-molecule structure, and shield lanthanide ion from
quenching and destabilizing effects of water. These compounds are characterized
by broad absorption spectrum in the range 240-320 nm with the peak at 266 nm.
Association of LC1 and LC2 with PC membranes was followed by the absorbance
increase, without any shift of maximum position. The observed dependencies of the absorbance
changes on lipid concentration were analyzed to quantify the drug redistribution between
aqueous and lipid phases in terms of partition coefficient. Partition coefficients for LC1 and LC2 were found
to be ca. 1.4×105 and 6.7×103, respectively. Allowing
for zwitterionic nature of PC molecules and the fact that lanthanide complexes
under study are highly hydrophobic compounds bearing no charge, it seems
reasonable to consider partitioning process as being driven primarily by
hydrophobic effect. It is tempting to suppose that different lipophilicity of the examined drugs originates from the
differences in their structures. To explore the drug effect on liposome structural properties we employed
pH-indicator dye bromothymol blue (BTB) and
fluorescent membrane probe pyrene.
At physiological pH
there exists an equilibrium between protonated and
deprotonated BTB forms. This dye responds to the changes in environmental
conditions by the shifts of its protolytic and
partition equlibria. The distribution of different
dye forms between aqueous and lipid phases is
determined by the properties of liposomal membranes and manifests itself in the
changes of BTB absorbance. Membrane association of different BTB species can be
quantitatively described in terms of the partition coefficients. As have been observed at low ionic strength partition coefficient of the protonated
dye form, is several orders of magnitude larger than of the deprotonated ones,
i.e. the extent of membrane association of the deprotonated form is negligibly
small. Due to high hydrophobicity of BTB species the
dye binding to membranes is driven mainly by non-ionic interactions. Incorporation
of Eu(III)
complexes into PC bilayer gives rise to increase of
partition coefficients of protonated BTB species, suggesting that these agents
can perturb membrane structure, presumably through generation of structural
defects and altering the conformation of PC headgroups. These findings were
further corroborated by pyrene excimerization
studies.
Emission spectrum of pyrene
monomers is featured by
a well-defined vibronic structure with five major vibronic
bands between 370 and 400 nm. Relative intensities of vibronic transitions
exhibit clear dependence on solvent polarity. The variations of the intensity ratio of the third to the first vibronic
bands (RIII) upon varying
drug-to-lipid molar ratio were found to lie mostly within experimental
accuracy. This implies that polarity of pyrene microenvironment remains
virtually unchanged on lanthanide incorporation into lipid bilayer, i.e. the
drugs do not affect the probe distribution across a lipid bilayer. In contrast
to the transverse pyrene location, lateral distribution of the probe undergoes
changes upon the drug addition. This is evidenced by the observed increase of
another parameter recovered from the pyrene spectra Ц excimer-to-monomer
fluorescence intensity ratio, which reflects the rate of probe lateral diffusion within membrane plane.
This parameter was found to increase in the presence of Eu
complexes suggesting decreased degree of lipid packing. Importantly,
the magnitude of all the observed bilayer-modifying effects appeared to
be rather small, the property, which, in combination with appreciable lipophilicity of the investigated compounds is favorable
for the development of liposome-based carriers of these potential anticancer
drugs.