Lipoxygenase in the Cells
of Angiosperms
Ewa
Szczuka1, Aleksandra Seta1, Marcin Domaciuk1,
Ewa Skórzyńska-Polit2, Irena Giełwanowska3
1Department
of Plant Anatomy and Cytology, Maria Curie-Skłodowska University,
Akademicka 19, 20-033 Lublin, Poland
2Department
of Plant Physiology, Maria Curie-Skłodowska University, Akademicka 19,
20-033 Lublin, Poland
3Department
of Plant Physiology and Biotechnology, University of Warmia and Mazury,
Oczapowskiego 1A, 10-719 Olsztyn, Poland
ABSTRACT
The enzyme lipoxygenase (LOX; EC
1.13.11.12) catalyzes dioxygenation of the long chain of fatty acids such as
arachidic, linoleic, and α-linolenic acids, which contain a cis,
cis-1,4-pentadiene structure. LOX has been found in all individual plant
parts of angiosperms. Its isoforms occur in the cells of seeds, pods,
seedlings, cotyledons, leaves, roots, fruits, young inflorescences, flowers
(e.g., in the cells of petals, anthers, in the walls of the microsporangium at
the stage of microspores and pollen grains in the loculus), young anthers,
microspores, pollen grains, and potato tubers.
The use of the immunogold labelling
technique allows precise evaluation of the localization of LOX on the
cytological level in the cells of angiosperm plants. Investigations with this
method have shown that LOX occurs in the cytosol (most often, the immunogold
particles were distributed randomly in the cytoplasm) and vacuoles. The
immunogold particles which revealed the presence of the enzyme were found to be
associated with microsomal membranes and the plasma membrane and were also
discovered in the close vicinity of mitochondria and near or within plastids (they were visible in the
area of the prolammellar body) and chloroplasts. In the latter, LOX was found
both in the envelope and in the stroma. Often, LOX was detected near (close to)
the short endoplasmic reticulum elements – mainly RER (rough endoplasmic
reticulum). Some single immunogold particles were observed at or in the area of
the cell walls of all the investigated parts of angiosperm plants. LOX was also
detected in the inner exine of the pollen grain and in places connecting exine
layers of neighbouring pollen grains.
Immunolocalization of lipoxygenase in an electron microscope
indicates a functioning „lipoxygenase pathway” in all cells of the investigated
angiosperm plant parts. The intensity of the immunogold reaction may indirectly
indicate differentiated activity of the enzyme in particular plant cells.
INTRODUCTION
Lipoxygenase (LOX; EC 1.13.11.12) is
widely spread in the cells of living organisms belonging to different
systematic groups. In plants, this enzyme catalyzes dioxygenation of the long
chain of fatty acids such as arachidic, linoleic, and α-linolenic
acids, which contain a cis, cis-1,4-pentadiene structure. Lipoxygenase
strongly prefers free fatty acids as substrates, but it has also been found to
have activity with polyunsaturated fatty acids (PUFAs) esterified to
phospholipids and neutral lipids such as triglycerides (Feussner and
Wasternack, 2002). Lipoxygenase plays a number of active roles in different
processes during plant life. These numerous and extremely important roles
decide, among others, even about the
construction of the LOX pathway. A possible LOX pathway has been shown in
Scheme 1.
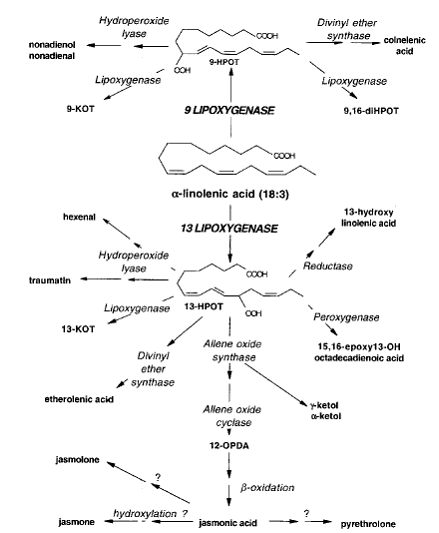
Scheme 1. The LOX
pathway. (The scheme was taken from Porta and Rocha-Sosa, 2002)
Lipoxygenase fulfils various functions in plants. In soybean, this
enzyme plays a role in nitrogen storage and partitioning. LOX can serve as a
vegetative storage protein, transiently storing nitrogen in the paraveinal
mesophyll cell layer prior to its redistribution to vegetative or reproductive
sink tissues (Feussner et al., 1997).
The enzyme in dry soybean seeds has a direct impact on the level of protease
inhibitors and their activity (Lima de Carvalho et al., 1999). LOX is believed to mediate the formation
of superoxide anion in senescent plants (Lynch and Thompson, 1984). Other
functions of LOX, including stress responses and pathogen defense, were
presented earlier by Rosahal (1996) or Porta and Rocha-Sosa (2002). The
numerous functions of LOX have been collected and arranged in Table 1.
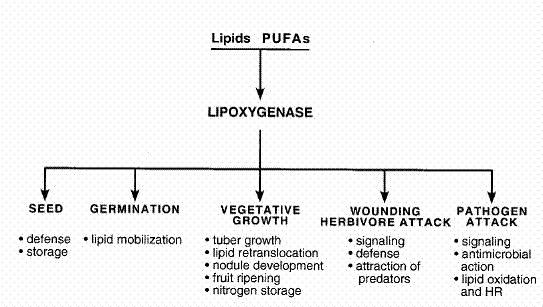
Table
1. Active roles of LOX in several processes during plant life. (The table was
taken from Porta and Rocha-Sosa, 2002)
Plant lipoxygenases are a frequent subject of study (for numerous
references see Porta and Rocha-Sosa, 2002). Despite of this only a few
investigators have focused on their localization. Meanwhile, the many functions
of lipoxygenase itself and the many
functions of the individual compounds of the LOX pathway seem to require for
their explanation, examination of the localization of this enzyme on the
cytological level, which can be done by electron microscopy and the immunogold
labelling technique. For instance, to our knowlege, there exist only a few
reports on LOX immunolocalization in different cells of plant organs (Wang et al., 1999; Leone et al., 2001: Szczuka et al.,
2006; Skórzyńska-Polit et
al., 2005; 2006). Therefore, in this paper we have focused our
attention on this, important for knowledge of cell and organ processes
occurring with the necessary presence of LOX.
MATERIAL AND METHODS
Plant material
Bulbs of Gagea lutea (L.)
Ker.-Gaw. (Liliaceae) growing in a natural habitat in Stalowa Wola
(south-western part of Poland) were used in the study. Both, flower buds and
leaves of Gagea lutea (L.) Ker.-Gaw.
were isolated from shoots growing from the bulbs under ground.
Light microscopy
Flower buds freshly excised from the bulbs and leaves cut into small
segments were fixed in a mixture of ethanol and acetic acid (3:1). After that,
they were washed in 70% alcohol, dehydrated, and embedded in paraffin wax. Then
the samples were cut into 4 μm specimens and mounted on slides.
Subsequently, they were stained with fast green and safranin (standard
procedure) and examined in a light microscope.
Small segments (2-3 mm) from flower buds and leaves were fixed in 3.5%
glutaraldehyde in 0.05 M cacodylate buffer, pH 7.0 for 24 h at room
temperature. The samples were postfixed in osmium tetroxide (OsO4),
dehydrated in ethanol and aceton, and embedded in Spurr’s resin. Semithin
sections of plant organs were stained with 0.1% toluidine blue in 0.5% sodium
carbonate at about 60°C.
Immunolabelling
For immunogold labelling, small segments (2-3 mm) from plant organs were
fixed in 2% formaldehyde (freshly prepared from paraformaldehyde) and 1%
glutaraldehyde dissolved in PBS (0.1 M phosphate buffer, pH 7.4) for 24 h at 4oC.
The samples were rinsed several times in PBS and 0.5 M NH4Cl in PBS,
dehydrated in ethanol, embedded in LR White resin (Sigma), and polymerised at
60oC overnight. Ultrathin sections were collected on nickel grids,
treated with aqueous 0.56 M sodium periodate for 30 min, thoroughly washed with
distilled water, and treated with 0.1 M HCl for 10 min followed by a 5 min
water wash. Sections were incubated first in 1 % BSA in PBS for 30 min at room
temperature, then with preimmune rabbit serum (Agrisera) diluted 1/1000 in
PBS-BSA for 1 h at room temperature. After triplicate washing with PBS-BSA
(each wash lasting 10 min), the sections were incubated with PBS-BSA containing
rabbit anti-LOX antiserum diluted 1/1000 for 1 h and repeatedly washed with
PBS-BSA. Goat anti-rabbit immunoglobulins conjugated to 10 nm gold particles
(GAR – gold) (Sigma) were diluted 1/50 in PBS-BSA and then applied for 40 min
at room temperature. Next, the sections were washed several times with PBS and
redistilled water. As an additional control, samples were incubated with
preserum and GAR-gold or GAR-gold only, omitting the primary antiserum. The
sections were stained with 2 % uranyl acetate for 5 min and Reynolds reagent
(lead nitrate and sodium citrate) for 1 min. All sections were examined using
the transmission electron microscope.
RESULTS
Lipoxygenase
was detected in different (organs) parts of the plants (Gymnosperms and
Angiosperms), but due to the limited scope of this article, we only focused on
parts of flower buds and leaves.
Gagea lutea
(L.) Ker.-Gaw. is an early-spring monocotyledonous plant. In Polish climate
conditions, it blooms in March. The morphological structure of the Gagea
lutea plant and its flower at the anthesis stage is shown in Figures 1 and
2. The single Gagea lutea plant grows
from a bulb (exactly from a shortened underground stem). The seedlings
containing stems, leaves and flower buds develop inside the bulbs and grow out
in early spring. Three different bulbs of Gagea
lutea with shoots growing above the bulbs are visible in Fig. 3. Flowers of
Gagea lutea have a structure typical
of monocotyledons. The centrally positioned pistil is surrounded by six stamens
and the same number of petals. In the mature flower, the stamen comprises an
anther inclusive of microsporangia and the intervening connective (Fig. 4), and
the filament. As shown in Fig. 4., the Gagea
lutea anther consists of four microsporangia. Each microsporangium is
surrounded by the anther wall, which is built of the epidermis, endothecium,
middle layer, and tapetum. In the loculus, microspores are present. Stamens and
anthers are enveloped by petals. Initially, a single stamen develops from the
primordium, which is built of mertistematic tissue. A transverse section of the
developing anther is shown in Fig. 5. The meristematic tissue of the future
microsporangia is lightly stained with toluidine blue. Such a developing anther
is surrounded by the petals (Fig. 6). Figures 3, 5, and 6 show the stages of
the development of leaves (Fig. 3), anther (Fig. 5), and petals (Fig. 6) which
were used to examine the localization of lipoxygenase with the electron
transmission microscope (TEM), shown in this paper.
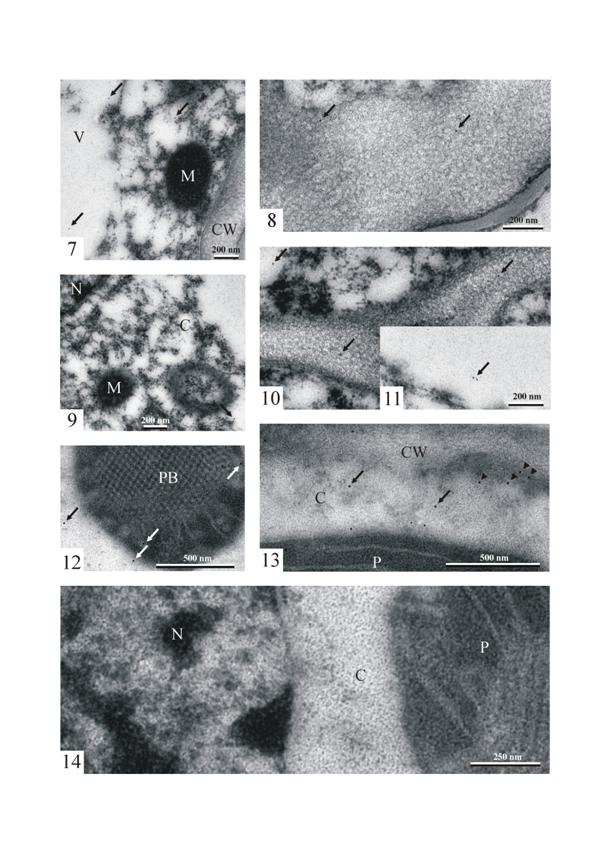
Immunogold LOX PAb localization in the
cells of the anther (at the stage of the young stamen) of Gagea lutea
shows the singular gold particles in the cytoplasm, in the vacuole (Fig. 7),
and in the area of the cell wall (Fig. 8). The density of the gold particles
that revealed the presence of lipoxygenase was very low. Similarly to the cells
of the young, developing stamen, the single immunogold particles were found in
the cytoplasm (Fig. 9), vacuoles, and the cell wall (Figs. 10 and 11) of petal
cells.
In a young leaf, 1-3 cm long, numerous immunogold particles were
observed in the dense cytoplasm with ribosomes of the parenchyma cell. The
particles were distributed randomly in the cytoplasm or gathered near short ER
elements and the outer plastid membrane (Figs. 12 and 13). Single immunogold
particles were visible in the close vicinity of mitochondria or small vacuoles.
Some immunogold particles were observed at the cell wall, organelle membranes,
or even inside the plastids, and in the area of the prolammellar body.
In order to determine the degree of specificity of the immunogold
reaction, a control reaction including all the procedures was carried out. The
control reaction was conducted omitting incubation with the primary antibody.
Only single gold particles (a few per one nickel grid) were found in the
specimens. In most grid meshes (like in the figure shown in this paper) no gold
particles were present (Fig. 14).
DISCUSSION
As it was mentioned in the introduction to this paper, plant
lipoxygenases are a very frequent subject of study (for numerous references see
Porta and Rocha-Sosa, 2002). The researchers have shown the occurrence of LOX
in plants using various methods. For example, the simplest method of LOX
localization is determination of the enzyme activity in individual plant parts.
The enzyme activity in a plant extract can be measured using (i) methods based
on oxygen uptake (manometric or polarographic techniques), (ii) methods based
on formation of conjugated diens, or (iii) determination of hydroperoxides
(Grossman and Zakut, 1979).
Another method used in the investigations concerned determination of
lipoxygenase isoenzymes is carried out by electrophoresis (SDS-PAGE, native
PAGE, IEF) (Grossman and Zakut, 1979; Heinisch et al., 1996; Smith et
al., 1997). Additionally, LOX activity can be determined by distinguishing
between lipoxygenase and heme proteins. Cyanide was suggested as a selective
inhibitor for distinguishing between them in the oxidation of fatty acids, but
according to Grossman and Zakut (1979) lipoxygenase activity is also sensitive
to cyanide. These authors cited another method of distinguishing the activities
of heme and non-heme proteins, which is based on the different effects of
linoleate on the fluorescence of these catalysts.
As reported in the Results section of
this paper, the localization of LOX was carried out by using the immunogold
labelling technique. This method (i.e. the immunogold LOX PAb localization method) allows to evaluate the
localization of LOX on the cytological level in the cells of all angiosperm
plants. For
example, the occurrence of lipoxygenase in different parts and types of anther
cells had been reveales with this method (Szczuka et al., 2004, 2006).
In
the cells of the developing Gagea lutea anther and petal, the immunogold LOX PAb localization shows the
presence of singular gold particles in the cytoplasm, in the vacuole and in the
area of the cell wall. Similarly, in both investigated developing parts of
flower buds the gold particles revealing
lipoxygenase were not numerous. This event indicates a low intensity of immunoreaction
and indirectly, a low activity of lipoxygenase.
In contrast, to the developing anther and petal in the flower bud, the immunogold LOX Pab reaction in a
young leaf was very intense. Numerous immunogold particles were found in the
dense cytoplasm of the parenchyma cell (mesophyll). Sometimes they were
distributed randomly in the cytoplasm, but very often they gathered near short
ER elements and the outer plastid membrane. In the mesophyll cells of young
leaves, single immunogold particles were visible in the close vicinity of
mitochondria or small vacuoles. Some single immunogold particles were observed
at the cell wall, organelle membranes, or even inside the plastids, and in the
area of the prolammellar body.
As it was mentioned earlier, the enzyme
LOX is widely spread in the cells of organisms belonging to different
systematic groups of plants, animals and fungi. In plant parts of angiosperms,
lipoxygenase or its isoforms occur in
young, developing organs, in mature organs, and also in degenerating parts of
plants. The presence of lipoxygenase was observed in the cells of seeds, pods,
seedlings, cotyledons, leaves, roots, fruits, young inflorescences, flowers
(e.g., in the cells of petals, anthers, in the walls of the microsporangium at
the stage of microspores, and pollen grains in the loculus), young anthers,
microspores, pollen grains, and potato tubers.
Additionally, our observations are partially supported also by results obtained
by other authors. For instance, Feussner et al. (1995) localized the enzyme
within the chloroplast using immunocytochemical analysis. Lipoxygenase
associated with the thylakoid membrane was found in tomato fruits (Bowsher et
al., 1992). LOXs were localized in the stroma, and substantial LOX activity was
detected in the chloroplast envelope fraction. In cotyledons, besides soluble
LOXs, particulate LOXs were also found in microsome membranes, plasma membranes
and lipid bodies (Feussner and Wasternack, 2002). Importantly, the above
results on LOX localization were only of marginal interest to the mentioned
authors. It should also be emphasized that LOX localization in cells is very
problematic, largely because soluble lipoxygenases tend to adhere to membranes
nonspecifically (Siedow and Girvin, 1980).
As shown in this paper,
immunolocalization of lipoxygenase in an electron microscope indicates a
functioning „lipoxygenase pathway” in all cells of the investigated angiosperm
plant parts. The intensity of the immunogold reaction indirectly indicates
differentiated activity of the enzyme in particular plant cells (Szczuka and
Skórzyńska, 2008). In the plant cells investigated in this paper,
the intensity of the immunogold reaction was comparatively low. Therefore, we
may assume that the activity of lipoxygenase in the tissues of the investigated
young plant organs in comparison to the activity of lipoxygenase in mature
plant organs is relatively low. Nevertheless, we would like to underline, that
at the moment knowledge concerning LOX localization on the cytological level is
still insufficient and further investigation is necessary.
ACKNOWLEDGEMENTS
We
would like to thank producer for developing antibody used in the experimental
part of this paper. Polyclonal antibody against antigen LOX was
produced by Agrisera, SE-911 21 Vännäs, Sweden, www.agrisera.se
LITERATURE
Bowsher C.G., Ferrie B.J.M., Ghosh S., Todd
J., Thompson J.E. and Rothstein S.J. 1992. Purification and partial
characterization of a membrane-associated lipoxygenase in tomato fruit. Plant Physiology, 100: 1802-1807.
Feussner I. and Wasternack C. 2002. The lipoxygenase
pathway. Annu. Rev. Plant Biol., 53: 275-297.
Feussner I., Hause B.,
Vörös K., Parthier B. and Wasternack C. 1995. Jasmonate-induced
lipoxygenase forms are localized in chloroplasts of barley leaves (Hordeum
vulgare cv Salome). Plant J., 7: 949-957.
Feussner I., Balkenhohl T.J., Porzel A., Kühn H.
and Wasternack C. 1997. Structural elucidation of oxygenated storage lipids in
cucumber cotyledons - implication of lipid body lipoxygenase in lipid
mobilization during germination. J. Biol.
Chem., 272: 21635-21641.
Grossman S. and Zakut R. 1979.
Determination of the activity of lipoxygenase (lipoxidase). Methods Biochem. Anal., 25: 303-329.
Heinisch O., Kowalski E., Ludwig H. and Tauscher
B. 1996. Staining for soybean lipoxygenase activity in electrophoretic gels. Fat/Lipids, 5: 183-184.
Lima de Carvalho W., Goreti de Almeida Oliveira M.,
Goncalves de Barros E. and Moreira, M.A. 1999. Lipoxygenases affect protease
inhibitor levels in soybean seeds. Plant
Physiol. Biochem., 37: 497-501.
Lynch D.V. and Thompson J.E.
1984. Lipoxygenase-mediated production of superoxide anion in senescing plant
tissue. FEBS Lett., 173: 251-254.
Rosahal S. 1996. Lipoxygenase in plants – their role
in development and stress response. Z. Naturforsch., 51c: 123-138.
Schmitt N.F. and van Mechelen J.R. 1997. Expression of
lipoxygenase isoenzymes in developing barley grains. Plant Sci., 128:
141-150.
Siedow J.N. and
Girvin M.E. 1980. Alternative respiratory pathway. Its role in seed respiration
and its inhibition by propyl gallate. Plant
Physiol., 65: 669-674.
Szczuka E., Skórzyńska-Polit E., Pawlikowska-Pawlęga
B., Sobieska J. and Gawron A. 2004. Localization of lipoxygenase in the anther of Gagea
lutea. Materials of 18th International Congress on Sexual Plant
Reproduction. Beijing (China)
2004: 53.
Szczuka E., Skórzyńska-Polit E., Pawlikowska-Pawlęga
B., Sobieska J. and Gawron A. 2006. Localization of lipoxygenase in the anther of Gagea
lutea. Acta Biol. Cracov., 48:
19-26.
Skórzyńska-Polit
E.,
Pawlikowska-Pawlęga B., Szczuka E., Drążkiewicz M. and Krupa Z.
2006. Localization and activity of lipoxygenase in Arabidopsis thaliana
plants under heavy metal stress. Plant Growth Reg. 48: 29-39.
Skórzyńska-Polit E., Pawlikowska-Pawlęga B.,
Szczuka E, Plak A. and Melke J. 2005. Localization and activity of lipoxygenase
in Cd-treated seedlings of Phaseolus coccineus. Acta Soc. Bot. Pol. 3:
199-207.
Szczuka E. and Skórzyńska-Polit E. 2008. Localization of
lipoxygenases in higher plants (in press).
Leone A., Melillo
MT. and Bleve-Zacheo T. 2001. Lipoxygenase in pea roots subjected to biotic
stress. Plant Sci. 161: 703–717.
LEGENDS
Fig.
1. General habit of the Gagea lutea (L.) Ker.-Gaw. plant.
Fig.
2. Gagea lutea. Bud and flower at
anthesis stage.
Fig.
3. Three different bulbs of Gagea lutea
with shoots growing above the bulbs.
Fig.
4. A transverse section of a Gagea lutea
flower bud. Note the four microsporangia (M) of the anther enveloped with thin
petals (PL). Fast green and safranin staining. 270x.
Fig.
5. A transverse section of the developing anther. Note the meristematic tissue
of the future microsporangia. Stained with toluidine blue. 380x.
Fig.
6. Fragments of petals (PL) of a Gagea
lutea flower – transverse section. Stained with toluidine blue. 380x.
Figs.
7 – 14. Immunolabelling to lipoxygenase
in Gagea lutea.
Figs.
7 and 8. Immunogold LOX PAb localization in the cells of the anther (at the
stage of young stamen) of Gagea lutea. Note singular gold particles
(arrows) in the cytoplasm (Fig. 7) and in the area of the cell wall (Fig. 8). M
– mitochondrion, V – vacuole, CW – cell wall.
Figs.
9 – 11. A portion of a petal cell of Gagea lutea. Single immunogold particles (arrows) in the cytoplasm (C)
(Fig. 9), vacuoles, and the cell wall (Figs. 10 and 11).
Figs. 12 and 13. Portions of a
mesophyll cell. Note singular gold particles (arrows) near and in the plastid
(P) in the cytoplasm (C) (arrows), near RER (arrowheads) and in the area of the cell wall (CW). PB –
prolammellar body.
Fig.
14. A mespophyll cell. A control micrograph of the cytoplasm (C) with a
fragment of the plastid (P), and the nucleus (N).