Àinagul Òîleuova, Dauletkhan Smagulov
K.I. Satpaev Êazakh National Technical
University, 050013, Êàzakhstan,
Almaty
Studying aluminum angle of Al–Cu–Mn–Zr system phase diagram as a base
for obtaining refractory aluminum alloys
Introduction
Recently there have rather actively been studied metastable condensed
systems possessing a number of new physical-and-mechanical properties differing
from the properties of equilibrium systems. A special place among them there is
taken by nanostructured materials whose volume significant part make grains
boundaries. Traditionally non-equilibrium (amorphous, nano- and microcrystal)
states in aluminum alloys are obtained by methods of the melt fast quenching,
mechanical alloy-forming and others.
With new technologies development there are made demands for structural materials quality, particularly, to aluminum alloys with transition metals possessing high operational and special physical properties, such as refractoriness, plasticity, fracture toughness and some others. To achieve these aims there are improved methods of materials treating in both liquid and solid states. To the first group there are referred temperature-temporal treatment and the melt high-speed crystallizing; to the second one – intense plastic deformation and thermal treatment.
Fast-quenched aluminum alloys containing 0.05 wt% by weight transition
metals (Zr, Fe, Cr) showed their good advantage as a base for prospective
refractory granular alloys mainly due to forming in them oversaturated solid
solutions. However, obtaining oversaturated solutions by the method of
high-speed crystallizing is connected with great technical difficulties. In
this connection there arises the necessity to look for other methods of
obtaining necessary structural state and the outside effects, for example,
intense plastic deformation that would assist the forming of certain structural
states ensuring the necessary level of the abovementioned alloys operational
characteristics.
In the present work with the aim of determining the zone of
concentrations and temperatures at which there can be achieved the maximum
level of refractoriness, there has been
carried out the quantitative analysis of Al–Cu–Mn and Al–Cu–Mn–Zr systems phase
diagrams. With the help of Thermo-Calc program there have been calculated the
corresponding isothermal and polythermal sections of phase diagrams, as well as
determined the temperatures of liquidus
and solidus.
Experimental studies
The analysis of the
alloys chemical composition (Table 1) shows that type 1201 alloys have significantly higher copper concentration
but lower manganese and zirconium concentrations than in ALTEK alloy. This
difference in concentration of alloying elements defined the key difference of
these alloys structure and properties.
Table 1. Some deformable alloys composition
based on Al–Cu-Mn-Zr system
|
Grade |
Cu, at% |
Mn,
at% |
Zr,
at% |
Others |
|
D201 |
6.0–7.0 |
0.4–0.8 |
0.2 |
Ti |
|
12012 |
5.8–6.8 |
0.2–0.4 |
0.1–0.25 |
Ti,
V |
|
ÀÀ 22193 |
5.8–6.8 |
0.2–0.4 |
0.1–0.25 |
Ti, V |
|
ALTEK4 |
1.2–2.4 |
1.2–2.2 |
0.15–0.6 |
Sc, V |
1BSt, 2SSt 4784-97, 3specification of The Aluminum Association (USA) 4 RF pat.
No 2252975 (publ. 27.05.2005, Bull. No 15)
Adding zirconium to binary alloys is known to
lead to forming Al3Zr phase [1]. Zirconium is known to increase greatly the liquidus temperature in
binary alloys. The calculation shows that copper presence effects but little
the degree of this increase that is demonstrated by polythermal sections shown
in Figure 1, as well as the data presented in Table 2.
From Table 2 we can see that a mere addition of copper doesn’t
almost effect the alloy crystallizing character. In non-equilibrium conditions
of crystallizing manganese solubility in aluminum increases, and a ternary
compound formation is suppressed. That’s why in such alloys alongside with (Al)
there co-exist phases Al2Cu and
Al6Mn. After forming virgin crystals
(Al) there occurs separating phases Al2Cu and Al20Cu2Mn3 in the following reaction: L→(Al) + Al2Cu + Al20Cu2Mn3 at the temperature 547 °Ñ. With the further increasing of copper concentration there are not
observed significant changes.
Table 2. Parameters of Al–Cu–Mn–Zr system characteristic alloys crystallizing
|
Cu, at% |
tL,
°C |
tS,
°C |
Phases |
|
2 |
730 |
628 |
(Al) + Al20Cu2Mn3 + Al3Zr + Al6Mn |
|
5 |
731 |
576 |
(Al) + Al20Cu2Mn3+ Al3Zr |
Though in literature there are no data on building a diagram of 4-component Al–Cu–Mn–Zr system, phase zones distribution in the aluminum angle of this system in solid state can be predicted based on the existing information.
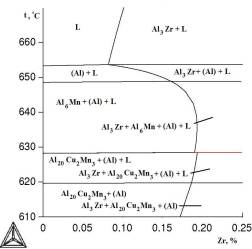
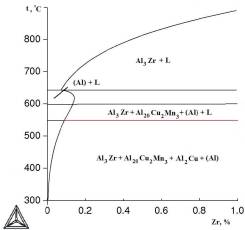
à
b
Fig.1.
Polythermal sections of Al–Cu–Mn–Zr system with varying zirconium
content: à) 2 at% Cu and 1.5 at% Mn; b) 6.5 at% Cu and 0.5 at% Mn
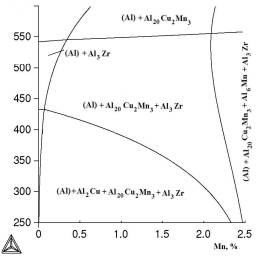
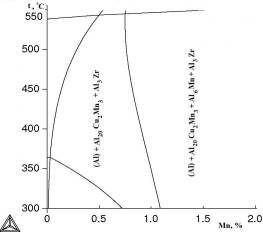
One of the most important
characteristics of any alloy is the liquidus (TL) and the solidus (TS)
temperature. With the help of these temperatures there are determined the modes
of thermal treatment, temperatures of alloys melting and casting. The results
of calculating TL and TS for some alloys of Al –Cu–Mn– Zr system are
shown in Table 2. Starting from the calculation results we can conclude that
copper doesn’t effect TL significantly but decreases TS obviously. On the other hand, adding 0.4 at% Zr already
increases the liquidus over 800 °C. The temperature effect on the phase zones location is shown on
the calculated polythermal sections with varying manganese content (Figure 2). It’s
obvious that with copper concentration decreasing from 2 to 1 at% there
decreases the probability of phase Al2Cu
forming. The
temperature effect is reflected by polythermal sections with varying manganese
content shown in Figure 2. Here we can see that copper content decrease from 2
to 1 at% decreases the probability of forming Al2Cu phase.
à b
Fig.2.
Polythermal sections of Al–Cu–Mn–Zr system with varying manganese
content: à) 2 at% Cu; b) 1 at% Cu: calculation for metastable phase Al3Zr (L12)
Conclusions
In the work based on Thermo-Calc program there
has been carried out the analysis of Al–Cu–Mn and
Al–Cu–Mn-Zr phase diagrams as a base for cast and deformable refractory aluminum
alloys.
To develop
refractory alloys designed for operating up to 350 0Ñ there
are suggested the alloys of Al–Cu–Mn–Zr system. As compared to industrial
alloys of 1201 type it is suggested to decrease copper content and to increase
manganese content. This will permit to obtain in the final structure the
maximum number of the secondary aluminides Al20Cu2Mn3
that (alongside with dispersoids Ll2) assist the hardening, especially at
increased temperatures. Besides, new alloys don’t require homogenization (as
the maximum plasticity is achieved in a cast state), that permits to decrease
significantly the deformed half-finished products cost.
References
[1] Ìînfoldo L.F. Structure and properties of aluminum
alloys. - Ì.: Ìåtallurgy, 1979.
[2] Belov N.A. Phase composition of aluminum
alloys: Scientific edition. – Ì.: MISiS publ. house, 2009. – 392 p.
[3] Belov
N.A., Alabin A.N. Prospective aluminum alloys with addition of zirconium and
scandium // Non-ferrous metals, 2007, No2, p. 99-106.
[4] RF patent No 2001145, Ñ22Ñ021/00. Cast alloy
based on aluminum / Belov N.A. Nov.15, 1993.
[5] Belov N.A. Structure and hardening of cast
alloys of Al – Ni – Zr system. Metal science and thermal treatment of metals.
1993, No 10, p.19–22.
[6] Belov N.A., Naumova Ye.A. Structure and
properties of cast alloys based on aluminum cerium system prospective
materials, 1999, No 6, p. 47–56.