Protsenko V.S.
Ukrainian State University of Chemical Technology, Dnepropetrovsk, Ukraine
Metal electrodeposition processes involving formation of stable
intermediates
Electrochemical reactions of metal deposition are of great technological
importance since they are the basis of all electroplating processes, as well as
many metal-winning industrial processes. When the electrochemical reaction involves
the transfer of more than one electron, it is usually considered that several
elementary steps are involved, and some of the intermediate valencies are
highly unstable [1]. However, in some instances, the intermediates may be
rather stable [2, 3]. For example, the electroreduction of Cr(III)
complex ions in aqueous solutions was demonstrated to proceed step-wise, via
the formation of relatively stable intermediates – Cr(II) compounds [4-6]:
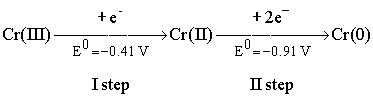
It was stated [4-6]that only a part of the total amount of Cr(II) complexes
formed at the first stage of Cr(III) discharge is reduced further to the metal.
Hence, the rate of the chromium deposition is determined by the current density
of Cr(II) ions electroreduction. The corresponding kinetic equations were derived
and compared with the experimental data obtained on a stationery and rotating
disk electrode. Kinetic parameters for the Cr(II) ions discharge were
calculated.
Analogues mechanism
was shown to be valid for the electrochemical reaction of iron
electrodeposition from chloride-citrate solution of Fe(III) salts [7]:
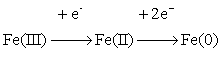
Iron electrodeposition begins upon reaching the
limiting current density of incomplete electroreduction of Fe(III) to Fe(II).
The Fe(II) complex ions are accumulated in the bulk of electrolyte. Hence, in
this case, the metal deposition also proceeds with the formation of
intermediates – Fe(II) compounds which are partially diffuse into the solution bulk.
Obviously, the equations deduced for the step-wise reaction of Cr(III)
electroreduction, can describe the electrochemical process of Fe(III)
electroreduction.
This work was also aimed to study the electrode processes occurring
during Cr-C electrodeposition from a trivalent chromium bath containing (mol dm-3):
0.5 Cr2(SO4)3×6H2O, 1 HCOOH, 1 CO(NH2)2,
0.15 Al2(SO4)3×18H2O, 0.3 Na2SO4,
0.5 H3BO3 and 0.1 g dm-3 of sodium dodecyl
sulfate [8-13].
Partial polarization curves of carbon
deposition was stated to replicate exactly (i.e. run strictly parallel) the ![]() -curves.
These results suggest that the rate of carbon co-deposition process is entirely
determined by the rate of chromium electroplating reaction. In other words,
electrochemical process of Cr-deposition imposes its own kinetics regularities
on carbon deposition.
-curves.
These results suggest that the rate of carbon co-deposition process is entirely
determined by the rate of chromium electroplating reaction. In other words,
electrochemical process of Cr-deposition imposes its own kinetics regularities
on carbon deposition.
We think that a part of active chromium ad-atoms generated as a result
of Cr(II) ions discharge may interact with the carbon of organic bath
constituent which is adsorbed on electrode surface [14]:
![]()
Carbon arisen enters into deposits structure providing nanocrystalline
structure formation. The rate of carbon co-deposition is influenced only by the
carbamide surface coverage and Cr ad-atoms concentration. The latter is
determined by the surface renewal speed that is by the partial current density
of chromium deposition ![]() .
.
Thus, the results of our investigations expand modern theoretical
conceptions on kinetics and mechanisms of electrochemical reactions proceeding
via several electron transfer steps. Additionally, the data obtained contribute
to solving important problem of electroplating processes rational organization.
References:
1. E. Gileadi, J. Electroanal. Chem. 532 (2002)
181-189.
2. V.S. Protsenko, F.I. Danilov, Russ. J. Electrochem. 41 (2005) 108-112.
3. F.I. Danilov, V.S. Protsenko, Russ. J. Electrochem. 40 (2004) 1-8.
4. V. Protsenko, F. Danilov, Electrochim. Acta 54 (2009) 5666-5672.
5. V.S. Protsenko, T.E. Butyrina, F.I. Danilov, Prot. Met. 43 (2007) 398-406.
6. F.I. Danilov, V.S. Protsenko, T.E. Butyrina, Russ. J. Electrochem. 37 (2001) 704-709.
7. F.I. Danilov, V.S. Protsenko, A.V. Ubiikon', Russ. J. Electrochem. 41 (2005) 1282-1289.
8. F.I. Danilov, V.S. Protsenko, V.O. Gordiienko, S.C. Kwon, J.Y. Lee, M.
Kim, Appl. Surf. Sci. 257 (2011) 8048-8053.
9. V.S. Protsenko, F.I. Danilov,
V.O. Gordiienko, S.C.Kwon, M.Kim, J.Y., Thin Solid Films 520
(2011) 380-383.
10. V.S. Protsenko, V.O. Gordiienko,
F.I. Danilov, S.C. Kwon, E-J. Chem. 8 (2011) 1925-1929.
11. V.S. Protsenko,
V.O. Gordiienko, F.I. Danilov, S.C. Kwon, M. Kim, J.Y. Lee, Surf. Eng. 27 (2011)
690-692.
12. V.O. Hordienko, V.S. Protsenko,
S.C. Kwon, J.-Y. Lee, F.I. Danilov, Mater. Sci. 46 (2011) 647-652.
13. V.S. Protsenko, F.I. Danilov, V.O. Gordiienko, A.S. Baskevich, V.V. Artemchuk,
Inter. J. Refract. Metal Hard Mater. 31 (2012) 281-283.
14. V.S. Protsenko, V.O. Gordiienko, F.I. Danilov, Electrochem. Commun. 17 (2012) 85-87.