Professor Evgeni V. Khmelevsky, professor Georgiy A. Panshin, professor Natalia Y. Dobrovolskaya, Yulia A. Ponkratova
Radiology
department, Russian Scientific Centre of Roentgeno-radiology, Moscow, Russia
Dose
reduction of postmastectomy irradiation in treatment of locally advanced breast cancer
Introduction
Anticancer activity
(in local control) of radiation following mastectomy has been known since the middle of XX century.[1] Nevertheless several randomized trials of EBCTCG[2] in 2005 studied
role of postmastectomy radiation in adjuvant treatment and revealed not only
14,8% reduction of local recurrence risk in patients with 4 and more metastatic
lymph nodes (median follow-up 5 years) but also 2,3% reduction of cancer-specific mortality (70,3% vs 68,0%) and 1,7%
reduction of overall (72,4% vs 70,8%) mortality (median follow-up 15 years).
Unfortunately this positive effect can be leveled by increasing of mortality
due to postradiation complications which can be even more severe if
chemotherapy and radiation combination is used. [3,4] G. Gagliardi et al. [5]
in 1997 reported that risk of death caused by cardiovascular
complications after radiation therapy of breast cancer vary from 2,1% to 12%;
they also demonstrated that the risk is significantly minimized by reduction of
dose cumulated by heart, this can be achieved by using 3D planning
techniques, protective blocks and
intensive radiation beam. Besides of
these methods one more can be proposed - adequate reduction of equivalent dose,
which is based on two preconditions:
1. improvement of dose
homogeneity in radiated areas due to upgrade of modern technologies;
2. additive effect of
modern neoadjuvant and adjuvant chemotherapy.
The letter method
is being investigated in our Centre among patients with locally
advanced breast cancer.
Material and methods
The study is pilot,
retrospective and nonrandomized. It includes 211 patients with II-III stages of
breast cancer treated with mastectomy and adjuvant radiation therapy in Russian
Research Centre of Roentgeno-radiology in 1998-2004. Median follow-up was 104 months. All patients were divided into 2
groups (according to dose to chest
wall):
1. 1 group –variant of
normal-dose (50 Gy to chest wall) radiation therapy with/without chemotherapy
(129 patients).
2. 2 group –variant of
low-dose (40 Gy to chest wall) radiation therapy with/without chemotherapy (82
patients).
Dose to regional
areas (46-50 Gy according to the number of metastatic lymphatic nodes) as well
as neoadjuvant and adjuvant chemotherapy regimes were similar in both groups.
Median follow-up
was 111 and 95 months for the 1 and 2 group respectively.
Treatment
As the first step
of treatment 128 patients were given neoadjuvant chemotherapy with CMF or CAF
regimens.
All the patients
had undergone radical mastectomy (Madden - 203
(95,8%), Halsted– 2 (0,9%), simple mastectomy – 6 (2,8%).
As
adjuvant treatment there were radiation
treatment, chemotherapy if recommended (4-6 courses of CMF, CAF or taxane-based
regimens) and
hormonal therapy in case of positive receptor status (in premenopausal women – LHRH
for 2 years or ovarioectomy with concurrent antiestrogens or aromatase
inhibitors during 5 years; in postmenopausal women - antiestrogens or aromatase inhibitors during 5 years).
Radiation
Radiation treatment
was performed with 1,2-6 Mev photons or high-energy electrons. In case of
photon radiation special fixing capacity and some methods improving dose
homogeneity in radiated volume were used. [6]
Statistics
Summary statistics
were conducted on personal computer using electron table «Microsoft Exñel» and packet of programs «Statistica for Windows»
v.6.0, StatSoft Inc (USA). Fisher and
Student exact tests were used as base criteria. Survival analysis was
estimated according to Kaplan-Meier method. Significance was defined as P value
≤0,05 . 95% confidence intervals and P values were calculated for each
parameter in both groups.
Results
Frequency of
several characteristics of patients’ condition, disease staging and treatment specialties
in both groups is presented at the
following Table 1.
Locoregional
recurrence
Frequency of
locoregional recurrence in 1 and 2
groups during all follow-up period was 6,2±2,1 % (8 patients) è 3,7±2,1% (3 patients) respectively. We
have found no statistically significant differences between two groups (p=0,4).
Mean time to relapse was 41 month (21-78 months) for the low-dose group and 21
months (9-34 months) for the normal-dose group, the difference is statistically
significant (p=0,003). From 8 cases
of locoregional recurrence in
normal-dose group 4 were local (3,1%), 2 – regional (1,6%) and 2 – locoregional
(1,6%); in low-dose group: 1 - local
(1,1%), 1 – regional (1,1%) and 1 – locoregional (1,1%) (Fig.1).
All recurrences in
the 1st group occurred in patients who got any adjuvant
chemotherapy, in the 2nd
group only one patient with relapse did not get it (so we could not
reveal any statistical dependence between locoregional relapse and adjuvant
chemotherapy, p=0,5). Adjuvant hormonal therapy also did not cause
statistically important changes: only 2 from 8 relapses in normal-dose group
occurred in patients who did not get adjuvant hormonal therapy (p=0,7) and all
3 relapses in low-dose group – in patients after it.
5-year frequency of
locoregional recurrence was 6,2±2,1% and
2,4±1,7% for 1 è 2 groups respectively (ð=0,6).
Distant
recurrence
Frequency of
distant progression in 1 and 2 groups during all follow-up period was 34,6±4,2%
(45 patients) è 19,5±4,4% (16 patients).
Low-dose radiation had
statistically
significant benefit (ð<0,05). Mean
time to distant metastases had occurred was 23 month (1-100 months) for the
low-dose group and 30 months (3-92 months) for the normal-dose group, the
difference is not statistically significant (p=0,7).
5-year frequency of
distant recurrence was 31±4,1% è 18,3±4,3% for 1 è 2 groups
respectively (ð<0,05).
Statistically
significant decrease of distant progression frequency in 2nd group
was more likely caused by lager number of patients with intact lymph nodes (26,5%
in low-dose vs 17,1% in normal-dose group).
Frequency of any
organs and tissues involvement in metastatic process did not differ
significantly between the groups. Patients from the 1st group had
metastases to liver in 55,6%, to bones – 48,4%, to lungs and pleura – 17,8%,
brain – 4,4%, nonregional lymphnodes and soft tissue – 20%. The same positions
in the 2nd group were 23,5%,
53%, 29,4%, 11,8%, 14,3% respectively.
We have found no
statistically significant influence of adjuvant chemo- and hormonal therapy on
distant recurrence frequency (ð=0,5).
Progression-free survival
There are no
statistically significant difference in progression-free survival (PFS) between
the two groups (Fig.2).
5-year PFS was
61,6±6,4% in the low-dose and 53,5±5,4% in the normal-dose group.
PFS occurred
to be in reverse relation to disease stage (table 2).
There were no
statistical difference in PFS between the groups depending on patients’ age,
number on regional lymphnodes involved and using of adjuvant chemo- and
hormonal therapy.
Overall survival
Overall survival
was not differ statistically between the two groups (ð=0,8) (Fig.3).5-year OS was 72,2±6,3% in the low-dose and 65,3±5,6% in the normal-dose group. We did not noted
any statistical differences between the groups according to the variant of
adjuvant drug treatment.
5-year cancer-specific
survival was similar for the normal-dose and low-dose groups: 78,2±4,8% and 79,7±6,1% respectively.
Late cardial dysfunctions
To evaluate the rate of late cardial dysfunction in the groups we used method of
pairs. From the whole number of patients we selected 2 groups of 20 women:
1st group - low-dose radiation treatment,
2nd
group - normal-dose radiation treatment.
As the
criteria for the forming groups we used (from more to less significance):
·
Localisation
of the tumor (left only)
·
Patient’s
age
·
Severity of
cardial pathology before treatment
·
Impairment
of cardac function during the course of treatment
·
Anthracyclines
in neoadjuvant and adjuvant chemotherapy
·
Comorbidities:
thyreoid disfunction, diabetes mellitus, obesity.
As a criteria of cardiovascular system statement only data from ECG was used. ECG is a routinely procedure before
and in the course of treatment as well as at least annually during follow-up
period. We considered cardiopathies both first revealed during the treatment
(as early complications) and during follow-up period (as late complications –
at least 1 year after start of treatment).
Late cardiac impairment on ECG occurred in 55±11,1%
and 75±9,7% of patients from low-dose and normal-dose group. We did not get
statistical difference, but strong tendency to more favourable results in
low-dose radiation can not be ignored.
Discussion
Results of our work
correlate to data from randomized studies estimated the influence of adjuvant
radiation therapy on progression-free and overall survival of patients with locally
advanced breast cancer (Table 3).
So, frequency of
locoregional relapse in our study was 3,7% and 6,2% for low-dose and
normal-dose variants of radiation, in other studies – 7-15% [7, 8, 9,10, 11, 12,
13, 14, 15]. The difference is likely to be explained by fewer number of
patients and shorter follow-up period in this work. 5-year progression-free survival also corresponds to world
statistic data: 64% and 44% for the 1st
and 2nd groups in our study and 48-69% according to foreign authors.
[9,10, 11, 13,15]
5-year
cancer-specific survival in both groups was 50% and 69,7%, 5-year overall
survival – 65,3% and 72,2% for normal-dose and low-dose groups respectively.
These results are not at variance with data from several studies of EBCTCG in
2005 where cancer-specific and overall survival accounted 70,3% and 72,4%.[2].
Data about
cardiotoxicity after postmastectomy
radiation vary significantly among different authors: from 1% to 54% [16, 17, 18, 19,
20].
According to study
conducted earlier in our Center frequency of late cardiac dysfunctions
registered on ECG estimated as 36,6±3,7%. In case of
cumulative dose on chest area less than 40 Gr or right-side tumors changes on
ECG were registered in 24%; and in case of left-side tumors – 32%. In the group
of patients who received cumulative dose on chest area more than 40 Gr changes
on ECG occurred in 36% and 50% in case of right-side and left-side tumor
respectively [21, 22, 23].
Preliminary results
of this study confirm influence of cumulative dose on frequency of
cardiopathies: late cardiac dysfunctions were registered with ECG in 55% in
low-dose group and 75% in normal-dose group (both in case of left-side tumors).
Summarizing all
afore-sited data, it can be concluded that all results of this method of
complex treatment including low-dose postmastectomy chest irradiation are comparable with other
researchers’ data, who used standard radiation therapy. Nowadays one of the
most important way of oncology development is treatment individualization.
According to this one of the main problem in radiation therapy of breast cancer
is development of methods of individual dose selection resulting in standard
level of local control and minimum of side effects. Our study is continued now
as a randomized research in order to solve afore-mentioned problem.
Table I.
Characteristic of some prognostic factors
|
Categories |
Normo-dose RT (1 group) Abs (%) |
Low-dose RT (2 group) Abs (%) |
ð |
|
Years ·
< 40 ·
41-50 ·
51-60 ·
61-70 ·
> 71 |
11 (8,5±2,5) 48 (37,2±4,3) 39 (30,2±4,0) 26 (20,2±3,5) 5 (3,9±1.7) |
6 (7,3±2,9) 23 (28,1±5,0) 24 (29,3±5,0) 29 (35,4±5,3) 0 |
ð<0,05 |
|
Local tumor (stage Ò) ·
1 ·
2 ·
3 ·
4 |
7 (5,4±2,0) 51 (39,5±4,3) 32 (24,8±7,6) 39 (30,2±4,0) |
5 (6,1±2,6) 37 (45,1±5,5) 24 (29,3±5,0) 16 (19,5±4,4) |
|
|
Regional lymph
nodes (pN) ·
0 ·
1 ·
2 ·
3 |
22 (17,1±3,3) 19 (14,7±3,1) 69 (53,5±4,4) 19 (14,7±3,1) |
21 (25,6±4,8) 14 (17,1±4,2) 36 (43,9±5,5) 11(13,4±5,5) |
|
|
Stage ·
IIA ·
IIB ·
IIIA ·
IIIB ·
IIIC |
6 (4,7±1,9) 14 (10,9±2,7) 50 (38,8±4,3) 36 (27,9±3,9) 19 (14,7±3,1) |
4 (4,9±2,4) 16 (19,5±4,4) 37 (45,1±5,5) 13 (15,9±4,0) 11 (13,4±3,8) |
ð<0,05 |
|
Drug treatment in
perioperation period ·
Neoadjuvant CT ·
Adjuvant CT ·
Adjuvant hormonal therapy |
86 (66,73±4,1) 113 (87,6±3,0) 100 (77,5±6,8) |
42 (51,2±5,5) 66 (80,5±4,4) 66 (80,5±4,4) |
|
|
Morphology ·
Ductal ·
Lobular ·
Ductal+Lobular ·
Special forms ·
N/A |
81 (62,8±4,2) 22 (17,1±3,3) 3 (2,3) ±1,3 5 (4,0±1,7) 12 (9,3±2,6) |
57 (64,0±5,3) 18 (20,2±4,4) 1 (1,1±1,1) 2 (2,2±1,6) 13 (14,7±3,9) |
|
|
Hormonosensitivity ·
Sensitive ·
Nonsensitive ·
N/A |
58 (77,3±4,8)* 17 (22,7±4,8)* 54 (41,8±4,3) |
58 (81,7±4,6)* 13 (18,3±4,6)* 11 (13,4±3,8) |
|
* of the
examined ones.
Table II.
Dependance of 5-year PFS on disease stage in the 1st and 2nd
groups
|
Stage RT |
IIb (%) |
IIIa (%) |
IIIb (%) |
IIIc (%) |
|
Normal-dose RT |
60,7±18,4 |
49±7,9 |
37,8±9,6 |
17,1±10,6 |
|
Low-dose RT |
58,5±17,2 |
65,6±9,4 |
65,1±18,7 |
40,8±18,0 |
Table III.
Effectiveness of postmastectomy radiation therapy (according to literature
data)
|
Authors, year |
Number of patients |
Decease stage |
Median follow-up |
Local relupse |
Distant relapse |
5-year PFS |
5-year OS |
Cancer-specific survival |
|
Dunst J et al., 2001[7] |
959 |
I-III |
10 years |
13,6% |
|
|
70,5
% |
|
|
Hehr T.et al., 2004[8] |
287 |
I-III |
5 years |
15,5% |
|
61% |
70% |
|
|
Yadav B.et al.,2007[9] |
688 |
I-III |
67 mon |
8,5% |
18,7% |
69% |
81% |
|
|
Fodor J.et al., 2003[10] |
249 |
T1-2N1 |
189 mon |
12% |
|
57%
(15-
year) |
52%
(15-
year) |
|
|
Chang D. et al., 2007[11] |
63 |
N3 |
15 years |
13% |
|
46% |
57% |
|
|
Wang SL. Et al., 2009[12] |
874 (<65 ëåò) |
IIb-IIIc |
47 mon |
0%
-IIb 7,2% - IIIc |
|
|
87% - IIb 79,2% - IIIc |
|
|
Overgaard M. et al., 1997[13] |
852 |
II-III |
10 years |
9% |
|
48% (10- year) |
|
|
|
Huang EH et al., 2004[14] |
542 |
>IIb |
10 years |
11% |
|
|
54%(10- year) |
58%(10- year) |
|
Zhang YJ. et al., 2009[15] |
217 |
T1-2N1 |
69 mon |
14,8% |
|
81,8% |
90,2% |
|
|
Our results (normal-dose RT) |
129 |
IIb-IIIc |
111
mon |
6,2% |
34,6% |
43,8% |
65,3% |
78,2% |
|
Our results (low-dose RT) |
82 |
IIb-IIIc |
95 mon |
3,7% |
19,5% |
64,0% |
72,2% |
79,7% |
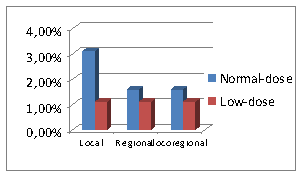
Fig.1.
Frequency of locoregional recurrence in
1 and 2 groups
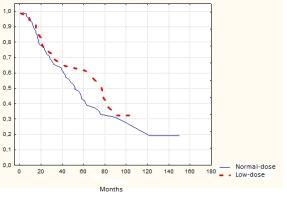
Fig.2.
Progression-free survival in the 1st and 2nd groups (p>0,05)
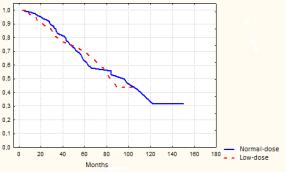
Fig.3. Overall survival in
the 1st and 2nd groups (p>0,05)
Conclusion
There are no statistically
significant differences in local control, overall and relapse-free survival
rates of traditional normal-dose and low-dose variants of postmastectomy
chest-wall irradiation. This method of dose-reduced postmastectomy chest-wall
irradiation in compliance with modern systemic treatment allows to decline risk
of cardiotoxicity with the maintenance of the treatment effectiveness achieved
by using traditional method.
Clinical Practice
Points
Anticancer activity
in local control of radiation following mastectomy has been known since the middle of XX century.
Then in 2005 several randomized trials of EBCTCG revealed also reduction
of cancer-specific mortality overall
mortality. Unfortunately this positive effect in survival can be leveled by
increasing of mortality due to postradiation complications. G. Gagliardi et al. in 1997 reported that
risk of death caused by cardiovascular complications after radiation therapy of
breast cancer is significantly minimized by reduction of dose cumulated by
heart.
In our study we proposed
method of dose reduction on chest-wall area (40Gr in 20 fr) which is based on
precondition of additive effect of modern neoadjuvant and adjuvant
chemotherapy. We got no statistically
significant differences in local control, overall and relapse-free survival
rates of traditional normal-dose (50Gr in 20 fr) and low-dose (40Gr) variants
of postmastectomy chest-wall irradiation, but strong tendency to more favourable results according to
cardiotoxicity in low-dose
radiation. One of the main problem in radiation therapy of breast cancer is
development of methods of individual dose selection resulting in standard level
of local control and minimum of side effects. We suppose that method of dose-reduced postmastectomy chest-wall
irradiation in compliance with modern systemic treatment allows to decline risk
of cardiotoxicity with the maintenance of the treatment effectiveness achieved
by using traditional method.
References
1.
Cuzick J, Steward H, Rutqvist L, et al. Cause-specific
mortality in long-term survivors of breast cancer who participated in trials of
radiotherapy. J Clin Oncol 1994;12:447-53.
2.
Early Breast Cancer Trialists Collaborative Group. Clarke M, Collins R,
Darby S, et al. Effects of radiotherapy and of differences in the extent of
surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized
trials. Lancet
2005;366(9503):2087-106.
3.
Giordano SH, Kuo YF, Freeman JL, et al. Risk of cardiac death after
adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005 Mar 16;97(6):419-24
4.
Gebski V, Lagleva M, Keech A, et al. Survival effects of
postmastectomy adjuvant radiation therapy using biologically equivalent doses:
a clinical perspective. J Natl Cancer Inst. 2006
Jan 4;98(1):26-38.
5. Gagliardi
G, Ingmar Lax, Gabor G. Prediction of excess risk of long-term cardiac
mortality after radiotherapy of stage I breast cancer. Radiotherapy and
oncology 1998. 46 (1): 63-71.
6. Khmelevsky E.V.
Radiation therapy of breast cancer. [in Russian].
Mammology. National guidance. Moscow; 2009:251-269.
7. Dunst J, Steil B,
Furch S, et al. Prognostic significance of local recurrence in breast cancer
after postmastectomy radiotherapy. Strahlenther Onkol. 2001 177(10):504-10.
8. Hehr T, Classen J,
Huth M, et al. Postmastectomy radiotherapy of the chest wall. Comparison of
electron-rotation technique and common tangential photon fields. Strahlenther
Onkol. 2004;180(10):629-36.
9. Yadav BS, Sharma
SC, Singh R, et al. Postmastectomy radiation and survival in patients with
breast cancer. J Cancer Res Ther. 2007;3(4):218-24.
10. Fodor J, Polgár C,
Major T, Németh G. Locoregional failure 15 years after mastectomy in
women with one to three positive axillary nodes with or without irradiation the
significance of tumor size. Strahlenther Onkol. 2003;179(3):197-202.
11. Chang DT, Feigenberg SJ,
Indelicato DJ, et al. Long-term outcomes in breast cancer patients with ten or
more positive axillary nodes treated with combined-modality therapy: the
importance of radiation field selection. Int J Radiat Oncol Biol Phys. 2007;67(4):1043-51.
12. Wang SL, Li YX, Song YW, et
al. Postmastectomy radiotherapy in moderate-and high-risk elderly breast cancer
patients. Zhonghua Zhong Liu Za Zhi. 2009; 31(11):863-6.
13. Overgaard M, Hansen PS,
Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women
with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer
Cooperative Group 82b Trial. N Engl J Med. 1997;337(14):949-55.
14. Huang EH, Tucker SL, Strom EA,
et al. Postmastectomy radiation improves local-regional control and survival
for selected patients with locally advanced breast cancer treated with
neoadjuvant chemotherapy and mastectomy. J Clin Oncol. 2004;22(23):4691-9.
15.
Zhang YJ, Sun GQ, Chen J, et al. Postmastectomy locoregional recurrence
and survival in early stage breast cancer patients with one to three axillary
lymph node metastases. Ai Zheng. 2009;28(4):395-401.
16.
Baysogolov G.D. Actual problems of radiation therapy.
[in Russian]. Medical radiology 1987; 3:3-6.
17.
Baysogolov G.D., Kirushkin V.I. Results of
electrocardiographical examination in patients with chronic radiation sickness.
[in Russian]. Newsletter of radiation
medicine 1961; 4:143-150.
18.
Baysogolov
G.D., Kirushkin V.I. Heart function in patients with chronic radiation sickness
in different decease periods (according to ECG data). [in Russian]. Radiation
and risk. Obninsk; 2000:43-47.
19.
Ivanickaya
V.I., Kislichenko V.A., Gerishtein I.G. et al. Complications of radiation
therapy in cancer patient. [in Russian]. Zdorovie. Kiev; 1989:181.
20.
Koritova L.I., Hazova T.V., Zhabina R.M. Radiation
therapy of locally advanced and
metastatic breast cancer. [in Russian].
Practical oncology 2000; 2:46-56.
21. Sergomanova N.N. Postradiation dysfunctions
of cardiovascular system in complex treatment of breast cancer. [in Russian]. Candidate's thesis. Moscow;2005.
22.
Khmelevsky E.V. Modern radiation therapy in treatment of locally
advanced and relapsed breast cancer. [in Russian]. Doctoral
thesis. Moscow;1997.
23.
Online journal: Khmelevsky E.V., Dobreniekiy M.N., Sergomanova N.N. et al. Risk factors of postradiation
disturbances in breast cancer patients. [in Russian]. Herald of Russian
research center of roentgenoradiology. Available at: http://vestnik.rncrr.ru/vestnik/v5/papers/hmel_v5.htm.
Accessed 2005.