ÓÄÊ 543.432: 543.645.6
Application of digital
colorimetry in control of phenols in building materials
Rudakov O.B.,
Khorokhordina E.A., Tran Hai Dang
Department of chemistry
of Voronezh State University of
Architecture and Civil Engineering, Russia
The opportunities of digital colorimetry in control of phenols in building
materials using color tests are considered.
Key words: digital
colorimetry, chromaticity parameters RGB, building materials, quality and safety control, phenols.
The opportunities of application of digital colorimetry in quality control
of colored building production are used more and more often because of the
development of digital techlogies [1-6]. From various models of representation of color we
chose the color scheme of RGB. The registration of a video signal by means of a
digital camera (DC) and by means of the flatbed scanners (FS) is used for the
development of colorimetric techniques. It is more convenient to use
DC in the field conditions, but too dark colors of coverings or solutions (if
it is a question of color tests for existence of ekotoxiñants) are undesirable to photoregistration. For DC it
is expedient to use a special box which allows unifying the photographing
conditions. At the same time, in order to obtain the images of solutions it is
possible to use not only ditches but also test-tubes with the help of DC. DC
allows photographing firm materials and products.
As for
FS, a scanner with a slide adapter is necessary for a registration of the
diluted solutions in big ditches. There are some restrictions on the sizes and
mass of a registered firm sample. A definite advantage of FS over DC are a
built-in system of lighting with automatic calibration of white balance and
sensitivity in each cycle of scanning.
The
image file received from DC or FS with the slide adapter, can be automatically
analysed by means of the standard software, both according to chromaticity
characteristics, and reflecting ability or lightness.
In
building industry phenols and their derivatives are used as antiseptics,
stabilizers and antioxidants, monomers and the precursors, though the lowest
phenols are ekotoxicants. Definition of their contents is particularly relavant
for a quantitative assessment of ecological safety of production. The most
selective control method of phenols is high performance liquid
chromatography (HPLC), and for an express control a thin-layer chromatography is also used [7]. In some cases when a
selective component wise definition is not required, the total content of
phenols (a phenolic index) is supervised by means of a spectrophotometric method [7].
A
number of color reactions for qualitative and quantitative definition of
phenols is known. They are applied in the analytical practice and the
diagnostics of materials [8]. The digital technologies give additional
opportunities for effective application of color reactions in the analytical
practice for a definition of phenols in water solutions.
For the analysis of building materials a sample of polymer was crushed
on the special crusher station (the sieve size 5×5 mm) and the mass of a
hinge plate was weighed on the analytical scales ~ 1,0-1,5 g with an accuracy of ±0,0002 g. Hinge plate was placed
into a conic flat-bottomed flask, 30 ml of the mixed solution an acetonotrile -
water in a volume ratio 4:1 were flowed with a graduated cylinder and stirred up with a vibratory blender within 15 min. 1,0 ml of the
received water-acetonotrilic extract was selected with a help of a pipette and
then was placed in a separating funnel, with a capacity of 50 ml.
10 ml of distilled water was added to this extract
with a help of a graduated cylinder, then we alkalinized with NH3
solution (C =1,6 mol/l) up to ðÍ 9 and
saturated this solution of sulfate of ammonium (NH4)2SO4
(~6,3 g). The received extraction system was stirred up with a vibratory blender within 15 min. After the
phase immiscibility (5 min) the photometric analysis with use 4 nitroanilines
was carried out.
For carrying out the reaction with chloride of iron (III) a reagent was
prepared, dissolving 5 g of waterless ferrous chloride (III) or 8,25 g of
ferric chloride hexahydrate (III) in 100 ml of distilled water. Extract of
phenols (0,5 ml) was added into 100 ml of water and then the reagent at the
rate of 0,1 g of a specimen on 0,05 ml of iron solution (III) was added. At the
time of addition of the reagent to the solution there was a violet coloring
[9].
After that there was a definition of chromacity by means of DC as
follows. To calculate the chromaticity coordinates and construct graduated
charts digital images of the analysed objects were received with the camera
Olympus SP-500 UZ in a special box which allows standardizing the lighting
conditions. A cuvette, filled with an experimental sample, was installed into a
box, and then the image was recorded. In order to reduce flare lights the
inside of the box was covered with matte black paint. A rear white side served
as a diffusion screen. For its lighting two halogen lamps with a total capacity
of 80 Watts were used. In the box there was a base for optical cells with
optical thickness from 10 to 100 mm. The optical scheme of the box allowed,
thanks to a turning mirror, having DC horizontally, making work of an operator
more convenient. Shooting conditions: save format graphical information – JPEG,
the image size is 1 MB, Flash-off, light sensitivity ISO 100 – or
"Auto", white balance settings – "halogen lamps".
Using
FS, the registration of digital images of the solutions was performed with a
scanner HP ScanJet 3500 with slide adapter in a specially constructed flask
clamp. The resultant images
were transmitted to a personal computer using the software FS. While scanning a
mode using the slide adapter was entered. The cuvette, filled with the analyzed solution, was placed into
the flask clamp, which consists of a light-tight box and a system of mirrors
and allows you to change the direction of illumination from the slide adapter
through the cuvette with a sample to an optical sensor of FS. The cuvettes were
with optical thickness l from the range of 5 to 100 mm. Scanning conditions:
TrueColor color mode (16.5 million colors) color scale RGB, 200 dpi optical
resolution.
The image file produced on a
digital device (DC or FS) was analyzed using its own program developed in an
environment of mathematical package Mathcad or electronic image editor Adobe
Photoshop.
In the paper [9] we present a method for
determining the content of individual phenols or the total content of their
mixtures in the aqueous solutions by analyzing the digitized images of the
solutions after two color reaction. The azocoupling reaction of phenols with
chromogenic agent was used as a color test. It was received by diazotizing para-nitroaniline (reaction 1), and the
reaction with FeCl3 (reaction 2). To obtain an analytical signal DC
was used.
The
objects chosen for a research were phenol, ortho-,
meta-, para-cresols, ortho-, meta-, para-dihydroxybenzenes and ortho-tret-butylphenol.
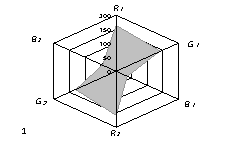
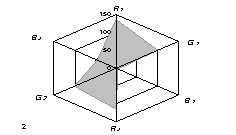
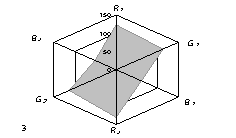
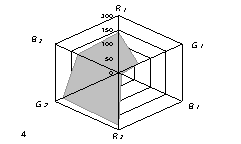
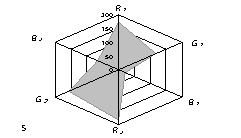
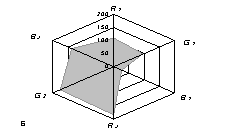
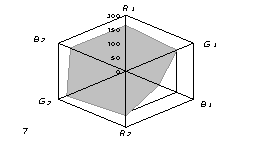
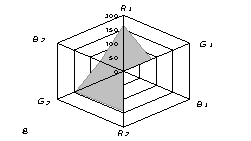
The generalized colorimetric data were presented in the form of the radar chart
(RC). On its axes there are Fi color coordinates in the RGB model in
a sequence of R1,G1, B1, R2, G2,
B2 where the indexes 1 and 2 are related to one of color reactions.
As can
be seen from fig. 1, RC form an individual profile ("a visual
print"), which is characteristic for each phenol which can be quantitatively
described using the RC geometrical parameters – area (S), perimeter (P),
fractality (D) and closeness value of
vector arrays ε [8].
RC area and perimeter can be considered as a
factor considering reactionary ability and structure of connection in case of
approximately identical concentration. The more intensive the received coloring
of solution is, the less the geometrical parameters of RC are, the better the
color reaction was carried out, or the stronger the chromophoric effect is which will depend on the balance
of mesomeric and inductive effects in the colored complex. So, if diatomic
phenols have 2 group -OH, both of them will react with a chromogenic reagent.
Alkyl
substituents, especially with a branched carbon skeleton, can complicate
sterically a color reaction in the ortho-position,
even a group –OH in the ortho-position,
forming a hydrogen bond with the adjacent group –OH, can impede the target reaction.
For
identification (PD/RC image recognition) fractality values (D) and a closeness value of vector
arrays (ε) were tested [9]. Values D and value ε to a lesser degree have to depend on the concentration of analyte, and
more characterize profile identity of a figure. The closeness value of vector
arrays ε appeared to be the most
informative.
In tab.
1 the RC geometrical parameters are sorted by the magnitude of the
hydrophobicity of phenols N which is equal to a logarithm of phenol
distribution between n-oktanol and water. The discovered trend – the higher the
hydrophobicity of phenol is, the more the area and the RC perimeter.
Table 1. Parameters of phenols hydrophobicity (H), and geometric parameters (area S, perimeter P,
fractality D and closeness value of
vector arrays ε) of colorimetric RC for different phenols, C = 0,15-0,19 g/l
|
Compound |
Í |
S |
P |
D |
ε |
|
meta- dihydroxybenzene |
0,80 |
19050 |
623 |
1,67 |
0,260 |
|
para-
dihydroxybenzene |
0,56 |
27250 |
657 |
1,78 |
0,215 |
|
ortho- dihydroxybenzene |
0,91 |
27940 |
740 |
1,81 |
0,248 |
|
meta-cresol |
2,00 |
32370 |
775 |
1,66 |
0,430 |
|
para-cresol |
2,13 |
33010 |
778 |
1,27 |
0,302 |
|
phenol |
1,64 |
36270 |
813 |
1,82 |
0 |
|
ortho-cresol |
2,13 |
39820 |
866 |
1,41 |
0,170 |
|
ortho-tret-butylphenol |
3,35 |
53260 |
894 |
1,66 |
0,472 |
The
minimal sizes of RC have dihydroxybenzenes,
it is the effect of two hydroxyls, and moreover ortho-dihydroxybenzene is more
similar to the phenol, it gives less dark color that confirms the assumption of
the decrease of the OH-group reactivity capacity in ortho-position.
|
|
|
|
|
|
|
|
|
|
|
Fig. 1. RD of
different phenol: 1) phenol; 2) meta-
dihydroxybenzene, 3) para- dihydroxybenzene
4) meta-cresol, 5) ortho-cresol, 6) para-cresol, 7) ortho-tret-butylphenol,
8) ortho- dihydroxybenzene; 1,5£Ñ£1,9 g/l
RC of para- and meta-cresol are
slightly different from each other, but the ortho-cresol
and ortho-tret-butylphenol, do
provide the palest coloring of the solutions that can be easily explained by
the steric effect of the substituent.
Thus,
the color reactions of phenols because of the difference in a structure do not
lead to identical, but to various parameters of chromaticity, and the
coefficient characterizes these differences quantitatively.
In the examined comparison the fractality D, due to
their simple configuration of comparable figures, does not let to identify the
geometry peculiarities of RC and is not suitable as a as a generalizing identification indicator.
Photometric and colorimetric methods of analysis of
the solutions, not differing in stages of sample preparation, differ only in
the way recording the response. In the first case the optical density of
absorbed light is detected with the photocolorimeter, and in the second – on FS
or DC, total color distinction of the samples in unities accepted in a
particular color model. Having a database
of standard color samples, it is possible to exclude the subjectivity of a
color evaluation, which is characteristic for visual examinations. For the
analysis of color parameters both transparent and opaque samples, in a solid or
liquid state are suitable.
Thus, the developed colorimetric method for determining
the concentration of phenol in the aqueous solution has several advantages
compared with photocolorimetry and HPLC, to be exact, at comparable precision
of the definitions it is realized using less expensive equipment, it allows
analyzing more concentrated solutions, is more informative than
photocolorimetry, due to the increasing number of the registered analytical
signals and demand smaller number of operations to carry out. In addition,
digital colorimetry is implemented using low-cost digital devices and standard
software.
List
of references
1. O. V. Baydycheva, I.V. Bocharnikova, O.B.
Rudakov., V.V. Khripushin Primeneniye scanometrii v kontrole kachestva
otdelochnyh materialov // Nauchny Vestnik VGASU.
Seria: Phisico-chimicheskiye problemy stroitrl’nogo. - 2008. - N. 1. - P. 100-105.
2. A. Muller Okrashivanie
polimernyh materialov. – S-Pb.: Profession, 2007. – 280 p.
3. Soldat D.J., Barak Ph., Lepore B.J. Microscale Colorimetric Analysis Using a
Desktop Scanner and Automated Digital Image Analysis // J. Chem. Educ. - 2009. - V. 86, N. 5. - Ð. 617-620.
4. Hirayama E., Sugiyama T., Hisamoto H., Suzuki K. Visual and Colorimetric Lithium Ion Sensing
Based on Digital Color Analysis // Anal. Chem. - 2000. - V. 72, N. 3. - Ð. 465–474.
5. V.M.
Ivanov, O.V. Kuznetcova. Khimicheskaya tcvenometria: vozmochznosti tcveta,
oblasti primenenia i perspektivy // Uspehi khimii. - 2001.
- V. 70. - N. 5. - P. 411-428.
6. I.V. Bocharnikova, O.B. Rudakov., E.A.
Khorohordina, V.V. Khripushin Primeneniye tcifrovyh technologiy v monitoringe
stoikosti oboev. // Stroitelnye materialy:
archetectura. - 2007, ¹ 9. - P. 28-29.
7. E.A. Khorohodina, E.A. Podolina, O.B. Rudakov Gidkostnaya
ekstraktcia smeshannymi rastvoritelyami. Primenenie v khimicheskom analize
fenolov. – LAP Lambert Academic
Publishing. - 2012. - 240 p.
8. Jork
H., Funk W., Fisher W., Wimmer H. Thin-Layer Chromatography. Reagents and
Detection Methods. - V.1. - VCN: New
York, 1990. - 497 p.
9. O.B.Rudakov, L.V. Rudakova, I.G. Kuduhova, P.A.
Golovinsky, E.A. Khorohordina,
E.N. Groshev Usovershenstvovanie sposoba opredeleniya fenolov po tcvetnym
reaktciyam s promeneniem tcifrovyh // Analitika
I kontrol’.- 2012. - V. 16. - ¹ 4. - P. 570-579.