R.Moradi, Yu.
Hozhiboev, N.Asadolahi
The synthesis 2-ph 6- R- amide deravatives 7-phenyl
5-oxo 5-H -1 ,3,4-thiadiazolo
[3,2-a] pyrimidine
V.I.Nikitin
Institute of Chemistry Academy of Sciences of the Republic of Tajikistan
Introduction
During recent years there have been intense investigations on
fused thiadiazole systems. Literature
survey revealed that [1,3,4] thiadiazolo[3,2-a]pyrimidine nucleus is associated
with diverse pharmacodynamic and
chemotherapeutic activities [1-10],
including antimicrobial [4,5,9,10]
and antitumor activities .
[1-4].
We
have synthesized 2-R 5-oxo 5-H 6-ethylcarboxamide 7-phenyl [1,3,4]thiadiazolo[3,2,-a]pyrimidine (F-J).Here,
we report using several process for the
synthesis of 2-R 5-oxo 5-H 6-ethyl carboxamide
7-phenyl 1,3,4-thiadiazolo[3,2-a] pyrimidine (F-J). in start cycloaddition reaction of oxalic
acide dichloride compound (A) and Ethyl
benzoylacetate (B) and produc 2- phenyl 4,5- dioxo 4-H-5-H,3- ethylcarboxilate furan (C)
(Scheme 1).
And then 2- phenyl 3-
ethylcarboxilate 4,5- dioxo 4-H-5-H, furan (C)
convert to ethyl 2- formyl 3- okco 3- phenyl propanoate (D) in boiling
dioxan (Scheme 2).
And
finally ethyl 2- formyl 3- okco 3-
phenyl propanoate (D) and 2- R 5-amino 1,3,4- thiadiazole (E) reacted to gether for synthesis 2-R5-oxo- 5H -6- ethyl carboxylate-7-pheny
-1,3,4-thiadiazolo[3,2-a] pyrimidine (F-J) (Scheme 3).
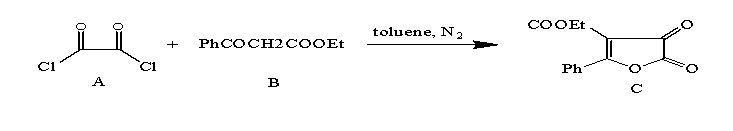
Scheme 1: synthesis
of 2- phenyl 4,5- dioxo 4-H-5-H,3- ethylcarboxilate furan
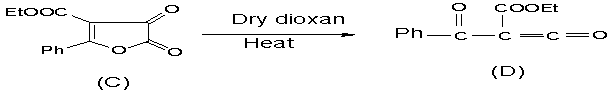
Scheme 2: 2-phenyl
3- ethyl carboxylate 4,5-dioxo 4,5-dihydro furan obtained
in boiling dioxan convert to ethyl 2- formyl 3- okco 3- phenyl propanoate
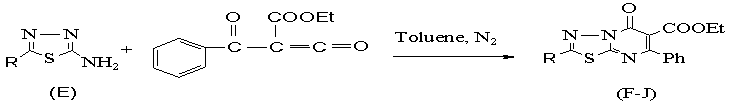
R:( H, CH3, Ph- , PhCH2-,
Br)
Scheme 3: synthesis
derivatives
of 2- R 5-oxo 5-H
6- ethyl carboxylate 7-phenyl
1,3,4-thiadiazolo [3,2- a]pyrimidine
In this
regard synthes of 2- Ph 5-oxo 5-H
6- R- amide deravatives 7- phenyl
-1,3,4-thiadiazolo [3,2-a] pyrimidine do
with The aim of 2- Ph
5-oxo 5-H 6-carboxylate 7-phenyl 1 ,3,4- thiadiazolo
[3,2-a] pyrimidine and deravatives amin in present solvent C2H5OH.
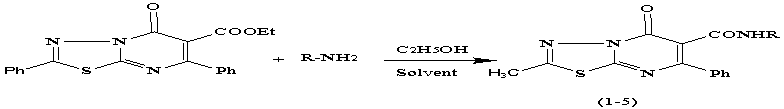
R: H(1) , -C2H5(2) , -(C2H5)2(3) , -NH2(4) , Morfolin(5)
The
composition and structure of the compounds( 1-5) are set elements analysis, IR,
1H NMR, 13C – spectroscopy.1HNMR 13C
- spectra of compound (1) when Phenyl has a molti signal corresponding duct located in the
second position, when (7.14-7.60) m. d. there is signal of proton amide
group in sixth position appears as a
singlet at 6,00 m.d.
The IR
spectrum of compound (1) in the crystalline state, the absorption band
is observed in the 1655cm-1 for the C = N- thiadiazole cycle
fragment, and the absorption band of C - S - C for C = N-thiadiazole cycle fragment
was
found in the area of 685cm-1.
The absorption band of C = O- amid
Fragment at 1660 cm-1. The absorption bands of N-H- amid Fragment at 3150- 3450 cm-1 and1550 cm-1 . and the band at 1715 cm –1 is characteristic of the carbonyl group in
fifth position cycle.
13C NMR - spectra of compound 2- Ph
5-Oxo 5-H 7-Phenyl
6- Carboxamide 1,3,4-thiadiazolo
[3,2-a] pyrimidine (1): 13C NMR (100 MHz, CDCl3,
δ ppm):118 (C), 126.4( CH ), 126,4 (CH), 127.6(c ), 128( CH), 128.7 ( CH),
128.7 ( CH), 128.9(CH),128.9(CH),129.2(CH),129.2(CH),130.7(c), 131.1(CH),
136.9(C),143.7( C ), 162,1(C) , 163 (C), 168 (C).
General
procedure for the synthesis 2-Ph 5-
oxo 5-H 6- R- amide deravatives 7- Phenyl
1,3,4 -thiadiazole [3,2-a] pyrimidine (1). A two-neck flask with
reflux condenser, magnetic stirrer
prevent 0.01mol, 2- Ph 5- oxo 5-H 6- ethyl carboxylate 7-phenyl
1,3,4-thiadiazolo [3,2-a] pyrimidine
10 ml C2H5OH . under constant stirring and added
portionwise R- amide deravatives (0.01 mole).The reaction was heated at 780C
for 10hours. Cool to room temperature, the precipitate filtered, washed with
dry ether and dried .
Ëèòåðàòóðà
[1]. A.T.
Taher, H.H. Georgey, H.I. El-Subbagh, Novel 1,3,4-heterodiazole analogues:
synthesis and in vitro antitumor activity, Eur. J. Med. Chem. 47 (2012)
ðð.445-451.
[2]. N.S.
El-Sayed, E.R. El-Bendary, S.M. El-Ashry, M.M. El-Kerdawy, Synthesis and
antitumor activity of new sulfonamide derivatives of
thiadiazolo[3,2-a]pyrimidines,Eur. J. Med. Chem. 46 (2011) ðð. 3714-3720.
[3]. M.B.
El-Ashmaway, M.A. El-Sherbeny, N.S. El-Sayed, Synthesis, in vitro
antitumor
activity and DNA-binding affinity of novel thiadiazolopyrimidine
and
thiadiazoloquinazoline derivatives, Mansoura J. Pharm. Sci. 26 (1)
(2010) 60e68.
[4]. M.A.
Mahran, M.A. El-Sherbeny, A.M.A. El-Obaid, F.A. Badria, Heterocyclic
systems
containing pyrimidine nucleus as potential antimicrobial and antitumor
agents,
Alexandria J. Pharm. Sci. 12 (1998) 39e44.
[5]. Y.
Tokunaga, S. Ito, Y. Kojima, S. Maeno, N. Sawai, Y. Sasao, Preparation
of2-phenylsulfinyl-5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-one derivatives as
agricultural
and horticultural fungicides, Jpn. Kokai Tokkyo Koho JP Patent 01
(83) (1989)
084.
[6]. S.
Herrling, 5-Carboxy-7H-1,3,4-thiadiazolo[3,2-a]pyrimidin-7-ones as immune
enhancers, Ger. Offen. Patent 2 (712) (1978) 932.
[7]. M.
Suiko, K. Maekawa, Synthesis and antitumor activity of 2-alkanesulfinyl (or
alkanesulfonyl)-7-methyl-5H-1,3,4-thiadiazolo[3,2-a]pyrimidin-5-ones,
Agric. Biol.
Chem. 41 (1977) 2047e2053.
[8]. K.
Maekawa, M. Mizumitsu, Thiadiazolo[3,2-a]pyrimidinone derivatives, Jpn.Kokai
Patent 77 (118) (1977) 494.
[9]. R.A.
Coburn, R.A. Glennon, Z.F. Chmielewicz, Mesoionic purinone analogues. 7.In
vitro antibacterial activity of mesoionic 1,3,4-thiadiazolo[3,2-a]pyrimidine-
5,7-diones, J. Med. Chem. 17 (1974) 1025e1027.
[10]. R.A.
Coburn, R.A. Glennon, Mesoionic purinone analogues. VI. Synthesis and in vitro
antibacterial properties of mesoionic thiazolo[3,2-a]pyrimidine-5,7-
diones and
mesoionic 1,3,4-thiadiazolo[3,2-a]pyrimidine-5,7-diones,
J. Pharm.
Sci. 62 (1973) 1785e1789.