Ecology/2. Ecological and meteorological
problems of cities and industrial zones.
Cand.tech.sc.
Stolyarova N. A., Chekhlan N. À., Chmykhalova Ju.Ju., Mikhailets E.À.
Automobile-Highway Institute of the State Higher Educational
Establishment «DonNTU», Ukraine
Lead-acid Batteries Recycling
Lead-acid batteries
have been widely used as autonomous chemical current sources for nearly 150 years. Today lead-acid
batteries firmly take the first place among all other kinds of chemical current
sources and there are still no alternatives to their use in vehicles and other
fields.
At the same time
exhausted batteries (service life up to 3 years) are ecologically dangerous. The reason for this lies in the toxicity of lead
contained in batteries (up to 60%
from weight) and chemical
aggressivity of acid electrolyte – sulfuric acid solution. Unfavorable ecological
situation forces to pay special attention to the problem of millions failed
batteries utilization and take measures to prevent harmful effect on the
environment and public health.
In
Ukraine the majority of exhausted batteries are taken abroad for recycling. So there is a need for modern plants for the lead-acid batteries utilization. As a provider of technologies and equipment we consider the popular
all over the world Italian company Engitec Technologies.
Technological
process of recycling begins with the discharge, collection and filtration of
the electrolyte. Batteries are thrown off by the crane into a concrete pit for depressurization.
The pit opens periodically to collect batteries released from the electrolyte. The
electrolyte is collected in the closed assembly by hermetic chutes. The
electrolyte is pumped into the electrolyte storage tank through a filter
trapping solid particles from it.
Batteries
released from the electrolyte are transferred by the crane to the receiver. Waste
batteries come to the conveyer belt that loads them into the crusher. The
magnetic separator is mounted over the conveyer for the metal contamination separation.
The crusher grinds waste batteries into pieces of 50-80 mm. They are loaded
into the separator where are washed by the flow of circulating water to
separate the lead paste. The lead paste consists of lead sulfate, lead oxides, and
particles of metallic lead. The paste is removed from the separator and collected
in a tank where is compacted. In the process of its obtaining plastic, polypropylene,
metal fractions are discharged.
To
remove sulfuric acid vapour and particles similar to dust a system of the
sanitary ware suction is provided. The air of sanitary ware suction passes
through the gas cleaner where it is washed by the filtrate flow.
At
a certain ratio components required for melting are fed into the loading
machine. It loads the obtained charge into the rotary furnace. Waste flue gases
and sanitary ware gases as well get to the precipitating dust chamber and bag
filter by gas ducts.
Melted
lead is poured into the furnaces where hardens. Then it is discharged in the
refining section. The resulting lead melt and its alloys are pumped to the
casting machine to obtain lead ingots and its alloys.
The
resulting slag is checked for lead and then is taken to the dump.
The
ultimate product is soft lead with the content of Pb>99,985%, as well as
lead and lead alloys.
Table 1 – Engineering-and-economic
performance of the production
|
Inputs name |
Material characteristic and energy supply parameters |
Measurement unit |
Yearly
consumption |
|
Lead-acid batteries waste |
Electrolyte
with the content of H PbSO PbO Metal
lattices -20+30% weight |
t |
26000 |
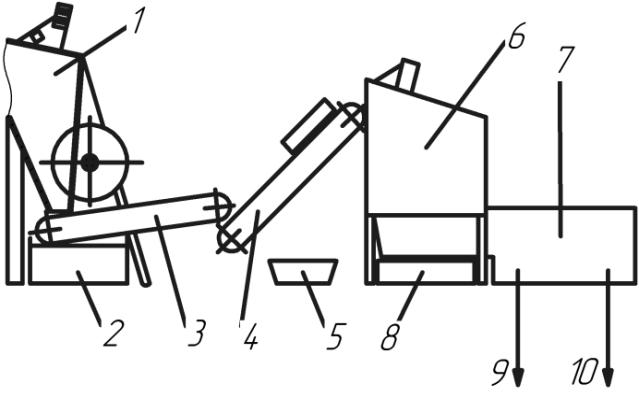
Fig. 1 – Schematic diagram of the operating
installation: 1 – products receiver; 2 – tank for acid collection; 3 – vibrational
unloader of damaged products; 4 – conveyer to move them to crush; 5 – magnetic separator; 6 – crusher; 7 – installation
of hydrodynamic separation of recyclable battery fractions; 8 – tank for lead
paste collection; 9 – polypropylene unloading; 10 – output of lead ingots and
its alloys.
Thereby, the given
schematic diagram allows to utilize batteries by the electrolyte
neutralization and the remelting of the lead recycling.
Literature:
2. http://www.recyclers.ru
3. http://revolution.allbest.ru
4. http://www.bibliofond.ru
5. http://www.energyarea.com.ua