ÁÈÎËÎÃÈ×ÅÑÊÈÅ ÍÀÓÊÈ / 6. ÌÈÊÐÎÁÈÎËÎÃÈß
Antoniuk S.Î., Sofilkanych À.P., Pirog T.P.
National University of Food
Technologies, Êyiv
DESTRUCTION OF AROMATIC COMPOUNDS BY THE
OIL-OXIDIZING BACTERIA NOCARDIA VACCINII Ê-8 AND ACENITOBACTER CALCOACETICUS ²ÌÂ
Â-7241
Up to 13 million lives could be saved each
year by reducing environmental risks, according to the World Health
Organization’s first country-by-country analysis of the impact of environmental
factors on human health. Environmental pollution is a great concern to all
countries around the world. China has made great efforts in this area and plans
to invest $175 billion in environmental protection between 2006 and 2010,
according to the National Development and Reform Commission of China [2].
Aromatic compounds are serious pollutants, being
present mainly in industrial wastewater from chemical, petrochemical,
pharmaceutical, textile and steel industries. Due
to their ubiquitous occurrence, recalcitrance, bioaccumulation potential and
carcinogenic activity, the aromatic compounds have gathered significant
environmental concern [3]. They are widely
distributed environmental contaminants that have detrimental biological
effects, toxicity, mutagenecity and carcinogenicity [6].
Although aromatic
hydrocarbons may undergo adsorption, volatilization,
photolysis and chemical degradation, microbial degradation is the major
degradation process. Bioremediation is the tool to transform the compounds to
less hazardous or nonhazardous forms with less input of chemicals, energy and
time [1]. Besides microbiological methods are economically advantageous, do not require large capital investments and operating costs, and local sewage treatment plants take low areas and very easy to maintain [3].
Numerous reports [1–6] have described the
ability of bacteria species to degrade aromatic and hydrocarbons such as Nocardia vaccinii and Acenitobacter calcoaceticus.
Previously it was shown that the bacteria Nocardia vaccinii K-8 and Acenitobacter calcoaceticus IMV B-7241 intensified the processes of oil
degradation in contaminated sites. Since crude oil always contains aromatic
hydrocarbons (10–50%), we assumed that the studied strains
may be potential destructors of aromatic compounds. In this
regard, the aim of our work was
to study the ability of strains IMV
B-7241 and K-8
to grow on nutrient media containing substrates of aromatic nature as a
carbon and energy source.
In the previous work the oil-oxidizing bacteria identified as Nocardia vaccinii Ê-8 and Acenitobacter calcoaceticus Ê-4 were isolated from the oil-polluted samples of soil. The ability of the
strains to synthesize the metabolites with surface-active and emulsifying
activity (biosurfactants) during their cultivation on different hydrophobic (n-hexadecane, liquid paraffin) and
hydrophilic (glucose, ethanol) substrates was determined. The strain K-4 was
deposited in the Depositary of microorganisms of the Institute of Microbiology and Virology of National
Academy of Sciences of Ukraine at the number of IMV B-7241.
A. calcoaceticus IMV
Â-7241
was cultivated on the nutrient medium of the following composition (g/L): NaCl
– 1.0; Na2HPO4 – 0.6; (NH2)2CO –
0.35; KH2PO4 – 0.14; MgSO4´7H2O – 0.1; ðÍ 6.8–7.0; the yeast autolysate – 0.5 % (v/v) and trace elements solution – 0.1 % (v/v) were also added.
N. vacinii K-8 strain was grown on the synthetic nutrient medium containing (g/L):
NaNO3 – 0.5; MgSO4´7H2O – 0.1; ÑaCl×2H2O – 0.1; KH2PO4
– 0.1; FeSO4´7H2O
– 0.1, yeast autolysate – 0.5 % (v/v).
Phenol, 4-chlorphenol, hexachlorobenzene, naphthalene,
benzoic, sulfanilic and N-phenilantranilic
acids 0.3–0.5 % (v/v), benzene and toluene 0.3–0.5% (w/v) were used as soul carbon and energy sources.
The variants of the inoculum were the following.
Variant 1 – 24-hour culture cultivated on the meat infusion agar (MIA). Variant 2 – culture cultivated on the liquid mineral medium
containing above-mentioned nutrients and aromatic compounds (0.1–0.25%) as soul carbon and energy sources.
The inoculum preparation included the adaptation of
bacteria to aromatic compounds
by gradual increase of their concentration from
0.1 to 0.25 % in
nutrient medium followed by bacteria inoculating on mineral medium with
0.3–0.5% of the substrate. For acclimatization a loopful of organisms cultivated on the MIA (24 h) was directly
inoculated into flasks containing aromatic compounds (0.1%) and all the
required nutrients. The culture was kept in a rotary shaker for 72 h.
This formed the primary culture. The secondary acclimatized
inoculum was prepared in the same way, wherein 10% (v/v)
of primary culture was used instead of the subculture to inoculate the medium
containing aromatic compounds (0.15%) and in this case the culture was incubated
for 48 h.
This was
continued for the third and fourth acclimatization by gradual increase
of aromatic compound concentration in nutrient medium to 0.2
and 0.25%, respectively. Variant 3 – 48-hour culture incubated in the liquid
mineral medium containing above-mentioned nutrients and aromatic compounds
(0.3–0.5%) as soul carbon and energy sources. The inoculum was used in a concentration of 10% (v/v).
The cultivation of bacteria took place in the 750 ml
Erlenmeyer flasks with 100 ml of medium on rotor shaker (320 rpm) at 28–30 ºÑ during 72–96 h.
The quantity of
synthesized surfactant was evaluated by such indexes: conditional surfactant
concentration (CSC*) and emulsification index (Å24, %) of the cultural liquid. The number of viable cells was
determined by the Koch method on MIA,
biomass – by the
optical density of cultural liquid, followed by recalculation to absolutely dry biomass by calibration graph.
It was determined that N. vaccinii K-8 and A. calcoaceticus IMV B-7241 intensively grew on phenol, hexachlorobenzene,
naphthalene, N-phenilantranilic and benzoic acid, slightly
worse on toluene, benzene and sulfanilic acid and died
on 4-chlorphenol.
The
utilization of aromatic
compounds accompanied by the formation of extracellular metabolites with surface-active
and emulsifying properties (Table).
Table. Surfactant synthesis during Acenitobacter calcoaceticus ²Ì Â-7241 cultivating on aromatic
compounds
|
Substrate |
Concentration, % |
CSC* |
Å24, % |
|
Phenol |
0.3 |
3.2±0,16 |
65±3,2 |
|
0.5 |
3.6±0,18 |
75±3,7 |
|
|
Benzene |
0.3 |
1.6±0,08 |
50±2,5 |
|
0.5 |
1.5±0,08 |
50±2,5 |
|
|
Toluene |
0.3 |
1.7±0,09 |
55±2,7 |
|
0.5 |
1.2±0,06 |
50±2,5 |
|
|
Benzoic acid |
0.3 |
2.1±0,1 |
55±2,7 |
|
0.5 |
2.8±0,14 |
52±2,6 |
|
|
N-phenilantranilic acid |
0.3 |
1.9±0,09 |
45±2,2 |
|
0.5 |
2.0±0,1 |
50±2,5 |
|
|
Hexachlorobenzene |
0.3 |
1.5±0,08 |
45±2,2 |
|
0.5 |
1.7±0,09 |
53±2,7 |
|
|
Ethanol (control) |
0.3 |
0.8±0,04 |
40±2,0 |
|
0.5 |
1.0±0,05 |
43±2,1 |
Note. The inoculum – 24-hour culture cultivated on the meat infusion
agar, the cultivation
took place during 96 h.
Thus, during A. calcoaceticus IMV B-7241 cultivation
on phenol (0.5%) the highest conditional surfactant concentration (CSC*) and emulsification
index (E24, %) were 3.6 and 70%, respectively (while on
ethanol CSC* – 1.0 and E24 – 43%).
The
maximum indexes of
surfactant synthesis by N. vaccinii
K-8 were observed as a result of strain growth on
the media with naphthalene (0.5%):
CSC* – 2.6 and E24 – 70%, while on
glycerol (0.5%) –
2.0 and 60%, respectively.
Similar
results were described by Nitschke et al. [4] testing polycyclic aromatic hydrocarbon degradation by Pseudomonas aeruginosa strains. So, P. aeruginosa N43
synthesized extracellular metabolites
with surface-active and emulsifying properties on phenantrene
(0.5%): conditional surfactant concentration and emulsification index were
3,3 and 75%, respectively.
Moreover, it was shown that the increased
concentrations of biomass (30–40 % of control) have been
observed in the case of three consecutive
inoculating of strains K-8 and IMV B-7241
on medium containing
aromatic compounds (0.3–0.5%).
Based on this
result, further the inoculum preparation included the adaptation of bacteria to aromatic compounds by gradual increase
of their concentration from 0.1 to 0.25 %
in nutrient medium followed by
bacteria inoculating in mineral medium with 0.3–0.5% of
the substrate. It was determined, that the process of cultivation was accompanied by 1.5–2.0-fold biomass increase
compared to using the inoculum cultivated on the meat infusion agar (figure).
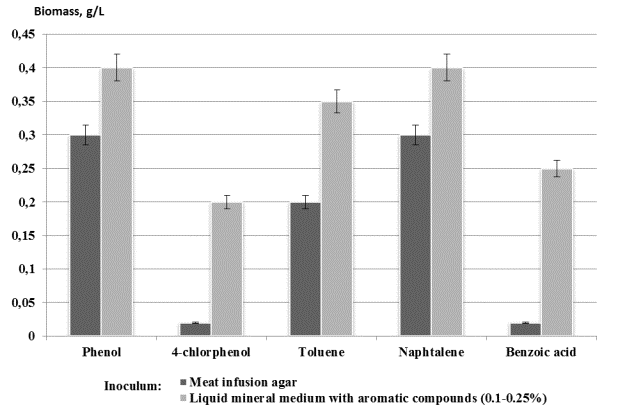
Effect of the inoculum
quality on growth of N. vaccinii K-8
on aromatic compounds (0.3%)
It
should be noticed, that in the case of the consecutive cultivation of the inoculum in liquid medium containing aromatic
compounds (0.1–0.25%)
biomass concentration of the strain K-8 was 0.4 g/L while Shetty et al. [5]
reported that biomass of Nocardia hydrocarbonoxydans on phenol (0.5%) was 0.25 g/L subject to use
above-mentioned method.
It
was determined, that N. vaccinii
K-8 and A. calcoaceticus
IMV B-7241 showed a particular
ability to assimilate aromatic compounds as soul carbon and energy sources and to synthesize practically
valuable surfactants. The growth of strains K-8 and IMV B-7241 on aromatic compounds was intensified in 1.5–2.0 times in the case of the consecutive cultivation of the inoculum on liquid mineral
medium with aromatic
compounds (0.1–0.25%) compared to using the inoculum cultivated on the meat
infusion agar. These strains are promising for use in the remediation of water and soil polluted with crude oil and aromatic
xenobiotics.
References
1. Baboshin M., Golovleva L. Multisubstrate kinetics of
PAH mixture biodegradation: analysis in the double-logarithmic plot //
Biodegradation. – 2011. – V. 22. – P. 13–23.
2. Daugulis A.J., Tomei M.C., Guieysse B.
Overcoming substrate inhibition during biological treatment of monoaromatics:
recent advances in bioprocess design // Appl Microbiol Biotechnol. – 2011. – V.
90. – P. 1589–1608.
3.
Haritash A.K, Kaushik C.P. Biodegradation
aspects of polycyclic aromatic hydrocarbons (PAHs): a review // J Hazard Mater.
– 2009. – Vol. 169. – P. 1 – 15.
4.
Nitschke M., Costa
S.G., Contiero J. Rhamnolipids and PHAs: reports on Pseudomonas-derived molecules of
increasing industrial interest // Process Biochemistry. – 2011. – V. 46. – P.
621–630.
5.
Shetty K.V., Verma D.K.,
Srinikethan G. Modelling and simulation of steady-state phenol degradation in a pulsed
plate bioreactor with immobilised cells of Nocardia
hydrocarbonoxydans // Bioprocess Biosyst Eng. – 2011. – Vol. 34, ¹1. – P.
45–56.
6.
Yang-Chun Y., Jian-Jiang Z. Review. Recent
advances in biodegradation in China: New microorganisms and pathways,
biodegradation engineering and bioenergy from pollutant biodegradation // Process
Biochemistry. – 2010. – Vol. 45. – P.1937 – 1943.