Chelbina Yu.V., Ilyin A.A., Tarabanko
V.E., Kaygorodov
K. L.
Institute of Chemistry
and Chemical Technology SB of RAS,
Akademgorodok 50/24,
Krasnoyarsk, 660036, Russia,
veta@icct.ru
INTERACTION
OF VANILLIN WITH CONCENTRATED SOLUTIONS OF SODIUM HYDROSULFITE
Oxidation of
lignosulfonates is applied to produce vanillin up till now [1-6]. Primary
purification of vanillin is carried out by hydrosulfitation and re-extraction
from the organic phase into an aqueous solution of sodium hydrosulfite [7]:
Ar-CHOorg + NaHSO3 aq = Ar-CH(OH)SO3Naaq . (1)
Quantitative data
on the stability of the bisulfite-vanillin derivative is extremely limited
(equilibrium constant of (1) Kobs = 350 ± 20, [NaHSO3]
< 1 M [8]).
The purpose of
this paper is to find methods to improve the efficiency of stripping vanillin
from the octanol phase with aqueous NaHSO3 solutions.
Experimental
Mixture of
vanillin in octanol and aqueous NaHSO3 (volume ratio Vorg:
Vaq = 10: 1) in a tube were shaken for 5 min. The organic phase
(octanol) was separated from the precipitate by filtration through a glass
filter, and vanillin concentration in octanol was determined before and after
extraction spectrophotometically.
Vanillin in the
precipitate formed in the stripping process was determined by GLC after acid
decomposition of vanillin bisulfate adduct and extraction. Vanillin content in
the aqueous phase after separation of octanol and precipitate of
vanillin-hydrosulfite-derivative was also analyzed by GLC.
The vanillin
distribution coefficient was calculated as the ratio of equilibrium
concentrations of vanillin in the aqueous and organic phases,
D = [Van] aq
/ [Van] org , (2)
were [Van] aq and [Van] org
- equilibrium concentrations of vanillin in the aqueous and organic phases,
respectively. In the case of three-phase system (when precipitate of
vanillin-hydrosulfite-adduct was formed), effective distribution coefficient of
vanillin from the organic phase was calculated, using the sum of the masses of
vanillin in the aqueous and solid phases, referred to the volume of the aqueous
phase Vaq instead of the equilibrium concentration in the aqueous
phase:
[Van]
aq = (m (Vanaq) + m (Vansolid)) / Vaq,
(3)
D
= (m (Vanaq) + m (Vansolid)) / (Vaq [Van] org),
(4)
The ratio (4)
characterizes the efficiency of vanillin stripping from the organic phase by
sodium hydrosulfite.
Results and Discussion
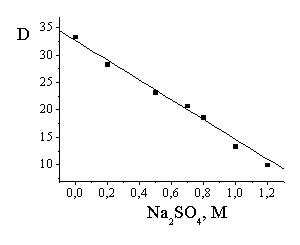
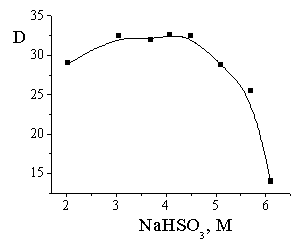
The ionic
strength (Na2SO4 solutions) strongly decreases the
distribution coefficient in two-phase system (Fig. 1). As a result of this
effect this coefficient decreases with [NaHSO3] increasing above 4 M
at low vanillin concentration (Fig. 2).
|
|
|
|
Fig. 1. Dependence of
the distribution coefficient versus
sodium sulfate concentration in the system 2.04 M solution of sodium
hydrosulfite – octanol. 0.066 M vanillin initial concentration in the organic
phase, Vorg / Vaq = 10:1. |
Fig. 2. Dependence of
the distribution coefficient of vanillin versus
sodium hydrosulfite concentration in the octanol-water system. 0.02 M
vanillin initial concentration in the organic phase, Vorg / Vaq
= 10:1. |
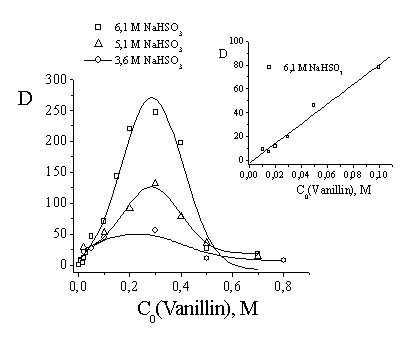
The
studied system becomes more complicated and efficient at high vanillin and
hydrosulfite concentrations as a result of precipitating the vanillin hydrosulfite
adduct (Fig. 3). In the field of saturated concentration of sodium hydrosulfite
(6.1 M), the dependence of the apparent distribution coefficient versus
vanillin concentration takes the extreme nature with Dmax values up
to 250 (Fig. 3). These values are 10 times more than the values of distribution
coefficient obtained previously at concentrations of sodium hydrosulfite below
1.2 M [8]. The most part of vanillin (appr. 90 %) was found in the precipitate
at NaHSO3 concentration 6.1 M, and this effect explains very high
observed distribution coefficient.
|
|
Fig. 3.
Dependence of apparent distribution coefficient of vanillin versus its
initial concentration in the octanol-water system. Process conditions:
initial sodium hydrosulfite concentration of 6.1 M (upper curve and its
initial part to the right), 5.1 M (middle curve), and 3.6 M (lower curve), Vorg
/ Vaq = 10:1. |
Thus, the vanillin hydrosulfite adduct was found to precipitate from
saturated sodium hydrosulfite solution, and it does not require additional
salting out agent, as is done in [9]. This effect reduces the consumption of
sulfuric acid and alkali for decomposition of the vanillin hydrosulfite adduct
down to the stoichiometric in opposite to the known methods [7] required 3 – 4
times more reagents.
REFERENCES
1. Diddams D.G., Krum J.K. In Kirk-Othmer
Encyclopedia of Chemical Technology. 2nd Ed., Interscience: New York,
1970, Vol. 21, P. 183-196.
2. Hocking M.B. Vanillin: Synthetic flavoring from spent sulfite liquor
// J. Chem. Educ., 1997, Vol. 74, P. 1055–1059.
3. Tarabanko V.E., Kaygorodov K.L., Koropachinskaya, N.V., Chelbina
Yu.V., Ilyin A.A. Production of aromatic aldehydes from waste biobutanol
production // Chemistry for Sustainable Development, 2012, Vol. 20, P. 471-476.
4. Taraban'ko, V., Koropachinskaya, N.; Kudryashev, A.; Kuznetsov, B.
Influence of lignin origin on the efficiency of the catalytic oxidation of
lignin into vanillin and syringaldehyde // Russian Chemical Bulletin, 1995,
Vol. 2, P. 375 - 379.
5. Tarabanko, V.; Koropachinskaya, N. Catalytic methods of producing the
aromatic aldehydes from lignins // Chemistry of plant raw Material (Russia),
2003, Vol. 1, P. 5-25.
6. Rodrigues Pinto P.C., Borges
da Silva E.A., Rodrigues A.E. Insights into Oxidative Conversion of Lignin to
High-Added-Value Phenolic Aldehydes // Ind. Eng. Chem. Res., 2011,
Vol. 50, P. 741-748.
7. Kamaldina O.D., Massov Ya.A. Production of Vanillin from
Lignosulfonates, TsBTI TsINIS: Moscow, 1959, 38 p.
8. Tarabanko V.E., Chelbina Yu.V., Kaygorodov K.L. The study extraction
of vanillin with octylamine and tributyl phosphate // Chemistry of plant raw Material (Russia), 2008, ¹ 4, P. 89-94.
9. Tcherniak J. Improvements in the Separation and Purification of
Vanillin. GB Pat. 268158, 1926.