Tarabanko V.E., Smirnova M.A., Chernyak M. Yu. and Morozov A.A.
Institute of
Chemistry and Chemical Technology, SB RAS,
Akademgorodok,
50-24, Krasnoyarsk, 660036, Russia,†† veta@icct.ru
ON THE REASONS OF SELECTIVITY
DECREASE OF THE CARBOHYDRATE ACID-CATALYZED CONVERSION
†††††††† One of the most common way to
process hexose carbohydrates is their acid-catalyzed conversion in water medium
to levulinic acid (4-ketopentanoic acid, LA) and 5-hydroxymetylfurfural
(5-hydroxymethyl-2-furaldehyde, 5-HMF). These compounds are used in different
fields including biofuel production [1 - 2].
The most important problem of the carbohydrate acid-catalyzed conversion
to LA and 5-HMF is to maintain the high selectivity with increasing the
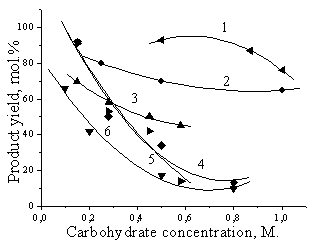
substrate concentration until 0.8 Ц 1 M. High 5-HMF yields (80 Ц 95 mol. %) are
obtained only in solutions of 0.1 Ц 0.5 M carbohydrate concentrations, and they
decrease dramatically at higher concentrations (Fig. 1) [1-4]. This problem is
extremely important from the industrial viewpoint and require to be interpreted
in terms of the reaction mechanisms.
|
|
|
Fig. 1 [4]. The carbohydrate
concentration influence on the yields of LA, 5-HMF and their derivatives in
different media (1 Ц fructose - water NaHSO4Ц butanol, 2 Ц
fructose - water Ц HCl, 3 Ц sucrose - water Ц HCl, 4 Ц fructose Ц butanol - H2SO4, 5 Ц sucrose Ц
water NaHSO4Ц butanol, 6 Ц fructose - water NaHSO4. |
The aim
of this paper is to study the influence of glucose and LA progressive additions
on the selectivity of the fructose acid-catalyzed conversion and to propose a
mechanism for the described above and discussed below data on the selectivity
decrease.
Experimental
Fructose
and glucose of food quality and levulinic acid of ACROS-ORGANICS (USA)† were used in the experiments. The
experiments were carried out in a 250 ml thermostated magnetically stirred
glass flask. The byproducts and intermediates were analyzed by GC-MS with Hewlett
Ц Packard GCD Plus spectrometer.
Results and
discussion
The effect of LA and glucose
addition on fructose conversion.†
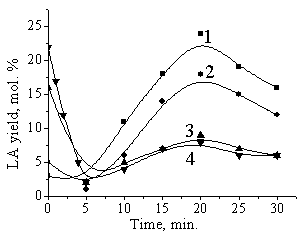
The
LA addition at the beginning of the fructose conversion greatly decreases the
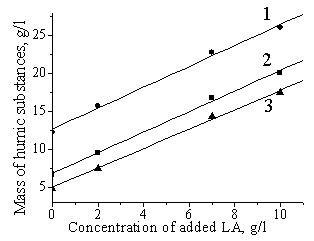
maximum yield of LA obtained in the process, favoring the increase of the humin
mass (Figures 2 and 3). There is a line dependence between masses of added
levulinic acid and humic substances formed (Figure 3), and tangent of these
straight lines is 1.5±0.1. In
|
|
|
|
Fig. 2. Influence of LA
additions on the LA yield in fructose conversion (0.4 M, 1080—, 2M NaHSO4, 1.7 M H2SO4.
The initial added LA concentration: 1 : without LA addition, |
Fig. 3. Influence of added
LA concentration on the yield of humic substances in fructose conversion (1080C,
2 M NaHSO4, 1.7 M H2SO4. The initial
fructose concentration: 1 : 0.4 M, |
all the
experiments, mass of humic substances increases with increasing fructose
concentration. These facts indicate that levulinic acid reacts with fructose
producing humic substances.
The
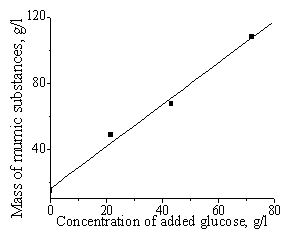
glucose addition similarly affects the fructose conversion (Fig. 4). The
influence of the glucose concentration on the humic substance yield shows
linear character (Fig. 5) and tangent of straight line is 1.3±0.1. This results
show that glucose reacts with levulinic acid giving rise to humic substances.
The
rate ratio of fructose to glucose conversion is approximately 20 Ц 30 [3,4].
Therefore glucose additives into the solution of converted fructose can not
detectably increase the overall LA yield, but purely demonstrate the formation
of humic substances from carbohydrates.
Comparing
the concentration data of Figures 2 and 4 shows that levulinic acid is
approximately 50 times more active in depressing the LA formation compared to
glucose. This means that high final concentration of levulinic acid, but not
high initial carbohydrate concentration in the process, principally limited
selectivity of acid catalyzed carbohydrate conversion.
|
|
|
|
Fig. 4. Influence of glucose
additions on the LA yield in fructose conversion (0.4 M, 1080—, 2 M NaHSO4, 1.7 M H2SO4.Glucose
concentration: 1 : without glucose
addition, 2 : 0.12 M, |
Fig. 5. Influence of added
glucose concentration on the humic substance mass in fructose conversion (0.4
M, 1080C, |
On the mechanism of humin formation. In order to
explain the obtained results and the just known data we suggest a novel
hypothesis on the mechanism of target products and byproducts formation
involving common carbocation species.
The
first stage of LA and carbohydrate conversion is proton accepting and formation
of the corresponding carbocations, immediately or after the removal of a water
molecule:
R2CHOH† +† H+† = R2CHO+H2† = R2C+H† + H2O
CH3C(O)CH2CH2COOH† +† H+† = CH3C+(OH)CH2CH2COOH
These
carbocation species can react then with the different molecules present in
solution. These interactions can be divided in two groups and therefore the two
possible pathways of carbocations conversion can be discussed:
(A)
Ц interaction with water molecule (or solvent molecule);
(B)
Ц interaction with substrate molecule or† molecular products of its conversion (5-HMF or LA etc.)
(A)
pathway of interaction with water molecules results finally in levulinic acid
formation [5]:
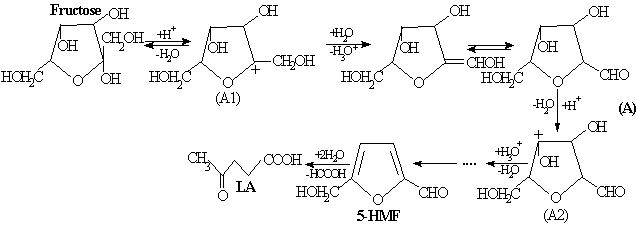
†††††††† (B)
pathway of carbocation interaction with reagent or target product molecules
results in increasing the product molecular mass, oligomerization and formation
of humic substances. For example, the interaction of the carbocation A1 formed
from fructose with enolic form of LA can give rise to ether derivatives of
higher molecular weight with respect to the starting reagents:
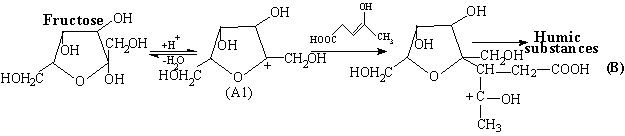
Thus,
selectivity of the acid-catalyzed conversion depends on the relative
contributions of (A) and (B) pathways. The relationship between these pathways
depends on the ratio of water to substrate activity, and, hence, levulinic acid
yield has to decrease with increasing the carbohydrate concentration in the
reaction mixture.
The
suggested mechanism allows to assume that in order to increase the selectivity
of concentrated carbohydrates acid-catalyzed conversion one have to extract
levulinic acid from the acid carbohydrate solution. This approach was
successfully realized by us adopting two-phase water-butanol system (Fig. 1)
[4].
†††††††† Acknowledgements.
Financial support from Russian Foundation for Basic Research (Grant No.
13-03-00754) is gratefully appreciated.
References
1.
Lichtenthaler F.W. Unsaturated O - and N - Heterocycles from
carbohydrate feedstocks// Accounts of Chemical Research. - 2002. - V. 35. - P.
728 Ц 737.
2.
Huber G.W., Iborra S., Corma A.† Synthesis of
transportation fuels from biomass: chemistry, catalysts and engineering//
Chemical Reviews. Ц 2006. Ц V. 106. Ц P. 4044 Ц 4098.
3.
Kuster B.F.M., van der Baan H.S. The influence of the initial and
catalyst concentration on the dehydration of D-fructose// Carbohydrate
Research. - 1977. - V. 54. - P. 165 Ц 176.
4.
Tarabanko V.E., Smirnova M.A., Chernyak M.Yu. Study of acid-catalyzed
conversion at presence of aliphatic alcohols at the moderate temperatures//
Chemistry for Sustainable Development (Russia). Ц 2005 - No 13 - p. 551-558.
5.
Antal M., Mok J.L., Richard G.N. Mechanism of formation of
5-hydroxymethylfurfural from D-fructose and sucrose// Carbohydrate Research. -
1990. - V. 199. - є 1. - P. 91 Ц 109.