Химия и химические технологии/8. Кинетика и катализ
PhD Viazovik V.
Cherkasy State Technological University
Optimization of
process of burning of gaseous fuel
The existent theory of geterogenious catalysis is
based on notion "thermal energy of activating". This parameter for
the flowline of concrete gas reaction represents the necessity of receipt by
the system (Gas – Gas) energy for overcoming power barrier. Bringing in the area of the promoted temperature to the catalyst (Gas - Gas – Hard system) changes reactionary power of the system. It is
necessary overcoming a few energies of activating the stages of catalysis,
algebraic sum of which considerably (at the correct choice to the catalyst)
below to energy of activating of gas reaction (Gas – Gas).
Using the catalyst, thus, gives the possibility to
intensify a process. But for flow lining the reaction in the system with a catalyst (for example, for
the gas, exothermic, reverse reaction) necessary is rising of temperature to
some one (enough high) level - "temperature of ignition " to the
catalyst. In most cases rising of temperature is energetically unprofitably and
economically not expediently. Practically, in all existent processes by
achievement reactionable level a temperature is basic. Activating of chemical processes is possible also with the use of
ultraviolet radiation, chemical processes in plasma and arc discharge in gases.
The fundamental scientific problem, which was put, is: to learn and
develop the apparatus and technology of electro-catalysis as the method for
declining the energy of activating on a catalyst due to bringing of him in the
area of quiet electric discharge. In the processes of electro-catalysis of
overcoming of energy of activating is carried out for the account of following
acts: synthesis and extinguishing of oxygencontained radicals; reception of
energoactive and reactionable atoms and molecules due to the stream of lone
electrons; wave influence of discharge on the system in an area to the
catalyst; ultraviolet irradiation; thermal influencing of quiet discharge.
Conduction of gas chemical reaction on a catalyst
in the area of quiet discharge intensification of process is going in after a
few directions:
-
oxidizing power of the system changes because as an oxidant not only oxygen but
also molecules of ozone (at low humidity) are used, and also (with the growth the
water pressure part) oxygencontained radicals НО![]() , НО
, НО![]() , RO
, RO![]() , RO
, RO![]() ;
;
-
molecules of reagent under action of high tension, stream of surplus electrons,
ultraviolet irradiation, e.t.c., grow into the energetically-excited atoms,
ions or ion-radicals;
-
oxidation of such reagents by oxygen, ozone and radicals flow spontaneously or
at the minimum of the energy charges;
-
compensation of energetic thermal charges is possible due to the rise of
temperature of the system in the area of discharge; it means using without
bulky heat-exchange
vehicles and caldrons;
-
influencing of frequency of discharge, optimization of strimmers working,
influence of temperature on a chemical reaction yield will be determined for
every system experimentally.
During the electro-catalytic activating of the systems
of burning (CnHm - N2 - O2) the
declining the energy of activating of the endothermic constituent on first stage of burning is achieved - hydrocarbons
decomposition on carbohydrate radical and proton. The decline expenses of
energy on the first stage of term destruction hydrocarbons of fuel leads to the
increase the selection of heat on the main heat-havier, that, in the turn,
results in the substantial economy of fuel (15-25%).
Achievement of high indexes of electro-catalysis is
related to the directed search of material of dielectric, laser treatment of
surface of dielectric, by the artificial extinguishing the radical of oxygen by steam of water,
studying of different constructions of ozonizers, as the reactors
of quiet discharge, studying of terms of synthesis of high concentrations of
oxygencontained radicals, prefiery preparations of fuel in fuel-air mixtures.
The
experiments on optimization of burning process of gaseous fuel were conducted
on stand and pilot options. For
prevention the declining of catalyst activity, a catalyst was shown out of area
of burning.
The experiments were conducted, both with a clean gaseous fuel, and with
addition in the gaseous fuel of different additives. As a fuel the methane
and propane-butane mixture were used. A
propane -butane mixture has the soft terms of electrocontacting comparatively.
Time of heating of definite volume of water was located from initial
(the temperatures 8-20 0C) to 98 0C. The volumes of
water made 1000 dm3. Every
experiment was repeated as minimally 3
times to reproduction of results. Researches were conducted at the expense of
the gas 150, 200, 300, 400 dm3/min (time of staying in the area of
electrocatalysis accordingly 0,036, 0,027, 0,018, 0,014 seconds).
The most economy of fuel is achieved at the dosage in the gas stream of
additives at tension 4-20 kV and arrived at approximately 12 %. Consumable
power at the electro-catalysis made on the average 3-5% from the got power due
to the economy of fuel. But
consumable power here considerably higher. On the economy of fuel time of gas
stream staying in the area of reaction
does not mean. On the fig. 1 dependences of water temperature change on time
during carrying out single test and experiment with a discharge are presented.
At by the use as a fuel natural gas the electro-initiation of process of
synthesis of radicals was carried out at tension of 10kV and higher. There was
the considerable economy of fuel ( without addition of additives ~15 %,
with addition of additives ~ 20 %).
The experiments on intensification of burning process of methane in the area of
electro-catalysis in the conditions of turbines at the transportation of
natural gas are conducted.
![]()
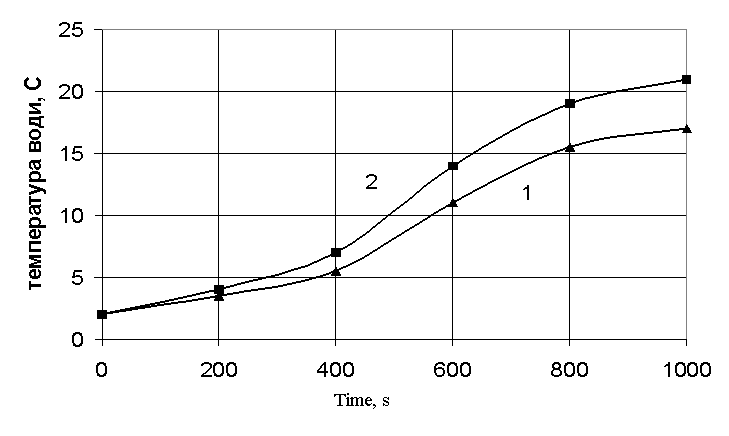
1 – idle
experiment; 2 - the alternative burning.
Fig 1 –
Curves of the dependence of water temperature change on time under the
conduction of a zero experiment and the experiment with a discharge