Chemistry and Chemical Engineering /
7. Inorganic Chemistry
Usoltseva N.V., D.Sc. Korobochkin
V.V.
Tomsk Polytechnic University, Tomsk, Russia
The Environmental Influence
of Substances
Obtained by Nonequilibrium Electrochemical Copper and Aluminium
Oxidation on Liquid-Phase Carbonization
It is
known that the heightened reactivity of nanomaterials given by high specific
surface area and uncompensated texture defects is
the reason of intensive interaction of nanomaterials with compounds
contained in the environment. In some cases, steps should be taken to prevent
adverse effect of environment on nanomaterial [1]. However, there may be
positive effect on material properties from interaction of nanomaterials with
such compounds.
At
present nanosized oxides and oxide systems are the focus of attention of
scientists. Nonequilibrium electrochemical oxidation is one of the promising methods
of metal oxide synthesis [2]. This method was used by us for copper-aluminium
oxide system synthesis [3].
Copper-aluminium
oxide system created by electrolysis interacts with dissolved carbon oxide during
ageing under solution forming basic carbonates [4]. As it was established
earlier there are not any adverse effects of basic
carbonates on characteristics of obtained oxide system [5]. On the contrary,
the pore structure characteristics of oxide system obtained from basic
carbonates are better than those of system created without carbonization. At
the same time uncontrolled variations of pressure, humidity of the air, content of
carbon oxide are the reason of fluctuation of composition of electrochemical
copper and aluminium oxidation product. XRD patterns of the interaction product
of copper-aluminium oxide system with compounds contained in the atmosphere at
the different atmospheric conditions are shown in the fig. 1.
At increased
humidity and carbon (IV) oxide content basic copper-aluminium carbonate (Cu-Al/LDH),
basic copper carbonate (Cu2(OH)2CO3) and boehmite
(AlOOH) are formed. The only basic copper-containing carbonate is formed under
conditions of decreased humidity and carbon oxide content. Meanwhile a part
of copper (I) oxide is oxidized to copper (II)
oxide in the solution. At present it is difficult to describe boundary
conditions that ensure the formation of basic copper carbonate. These
conditions depend on correlation of the above-mentioned characteristics
of environment.
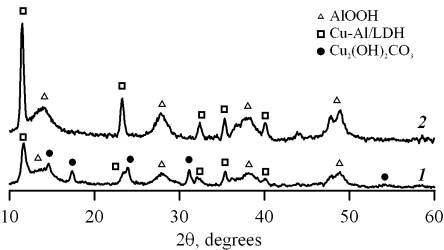
Fig. 1. XRD
pattern of products of electrochemical copper and aluminium oxidation formed
under conditions of comparative increased (1) and decreased (2)
values of air humidity and carbon (IV) oxide content
It
seems that not so mild conditions are necessary for
basic copper carbonate formation in comparison with basic
copper-aluminium carbonate. Interaction of semiproducts and products of metal
oxidation in conditions of electrolysis is believed to be the reason of existence of
basic copper carbonate or copper (II) oxide along with boehmite according
to air conditions. As a result there are two forms of copper (I) oxide in
the product of electrolysis that are more and less intensively associated with
boehmite.
Porosity
of material and its heat resistance strongly depend on crystalline structure. Hence
dependence of composition of oxide system precursor on atmospheric conditions
may be the reason of uncontrolled variation of pore structure characteristics
of oxide system.
Actually,
mixed metal oxides with high heat resistance, big specific surface area and mesoporosity
are formed by heat treatment of Cu‑Al/LDH. Such textural characteristics are favorable for the catalytic and
sorption materials [6]. Basic copper carbonate also has a positive
affect on properties of oxides obtained from it. However it is not obvious.
Thus,
we believe that there is sufficient reason for further research aimed at
support of permanency of atmospheric conditions that is in contact with product
of electrochemical oxidation. It should be mentioned that heightened reactivity
of nanosized product of electrochemical copper and alumunuim oxidation may be
the reason of product pollution by various compounds contained in the ambient
air.
The above-mentioned
restrictions allow talking about suitability of creation of induced environment
that has advantage of natural one (contain carbon oxide) and does not have
disadvantage (contain extraneous substances). Change
in humidity and pressure of air, content of carbon oxide gives an opportunity
to select conditions that are more suitable for basic carbonate formation than
those in natural environment.
In
spite of the
conclusion that it is necessary to avoid an interaction between
product of electrolysis and environment this study is very useful. It is due to
the opportunity
expanding of property change by gas
process. The fact that gas carbonization takes place in the mild conditions allows
believing that this method of improvement of electrolysis product is more
promising.
Literature:
1.
Fedorov S. G.,
Guseinov Sh. L., Storozhenko P. A. Nanodispersed
Metal Powders in High-Energy Condensed Systems // Nanotechnologies in Russia. – 2010. – Vol. 5. –
Nos. 9–10. – Pp. 565–582.
2.
Korobochkin V.V. Processes of Nanodispersed
Oxide Obtaining Using Alternating Current Electrochemical Oxidation of Metals: diss.
… doctor of engineering science.
– Tomsk, 2004. – 273 p.
3.
Usoltseva N.V., Korobochkin V.V. Alternating
Current Electrolysis as a Method of Copper-Aluminium Oxide System Synthesis // Materials of the Ist International Russian-Kazakhstan
Conference on Chemistry and Chemical Engineering. – April 26‑29, 2011. –
Tomsk: TPU Press, 2011. – Pp. 205–207.
4.
Usoltseva N.V. Investigation of Cu–Al–O System Carbonization in Air
// Abstracts of the
VIth National Conference of Young Scientists, PhD and Students
with the International Participation “Mendeleev–2012”. Inorganic chemistry. – April 3–6,
2012. – SPb.: Solo press, 2012. – Pp. 330–332.
5.
Usoltseva N.V., Korobochkin V.V. Thermal stability of
copper-aluminium oxide system precursors obtained by electrochemical synthesis
// Materials of the IInd
International Kazakhstan-Russian Conference on Chemistry and Chemical Engineering
dedicated to the 40th Anniversary of academician E.A. Buketov
KarSU. – Vol. I. – February 28 – March 2, 2012. – Karaganda:
Publishing House of KSU, 2012. – Pp. 259-261.
6.
Handbook of Layered Materials. Edited by Scott M. Auerbach,
Kathleen A. Carrado, Prabir K. Dutta. – N. Y.:
Marcel Dekker, Inc., 2004.
– 646 p.