ÕÈÌÈß È ÕÈÌÈ×ÅÑÊÈÅ
ÒÅÕÍÎËÎÃÈÈ/ 6.Îðãàíè÷åñêàÿ õèìèÿ
The
synthesis of 2,6-bis(phenil)hexahydro-1,3,5-triazine-4-thion
Ph.D.
A.R.Sujayev
Acad.
A.M.Guliyev Institute of Chemistry of Additives NASA, Azerbayjan,
Beyukshor str., 2062, AZ 1029, Baku, efsun_07@mail.ru
Abstract
By
three- component condensation of benzaldehyde, thiourea and 33.5% water
solution of ammonia has been synthesized
2,6-bis(phenyl)hexahydro-1,3,5-triazine-4-tion.
Keywords: benzaldehyde, thiourea
INTRODUCTION
According to the literature data 2,6-bis(phenyl)hexahydro-1,3,5-triazine-4-tiones
are the class of substances which shows antiseptic, bleaching, explosive and
other properties. A lot of their keto derivatives shows physiological activity.
For example, 5-azasitidin named by 4-amine-1,3,5-triazine-2-on ribofuranozed
shows anti- cancer and anti- leukemia features and it is used in medical
clinic. In the national industry 6-metylamino-1-metyl-3-cyclohexsyl-1,3,5-triazine-2,4-dion
or hexazinon used as a herbisid in destroying the weeds. 1,3,5-triazine
derivatives – melamine, melamineformaldehyde are used in synthesis
thermoplastic materials, but cyan chlorine in obtaining the paints.
Hexahydro-1,3,5-triazinetions are class of effective antioxidants that have
removed the oxidation of model carbohydrogen kumol.
EXPERIMENTAL
The synthesis of 2,6-bis(phenyl)hexahydro-1,3,5-triazine-4-tion.
11.4 g. (0.15 mol) thiourea, 20 ml isopropyl alcohol and 21.2 gr (0.2 mol)
benzaldehyde added to the three – neck flash which equipped with mechanical
stirrer, thermometer and drop funnel and dynamically mixed. Then 33.5% water
solution of ammonia added slowly to the reaction mixture. Mixed in 4 hours in
25°C temperature. Reaction process have observed with thin - layer
chromatography. When the reaction completed the mixture persisted during 24
hours at room temperature and white crystals precipitated. Crystals of 2,6-bis(phenyl)hexahydro-1,3,5-triazine-4-tion
filtrated and separated, then washed in dichloromethane and crystallized in
ethyl alcohol. Obtained 7.41 g. of 2,6-bis(phenyl)hexahydro-1,3,5-triazine-4-tion.
Yield 65%. M. P.= 232°C. Rf=0.54.
Found, %: C 66.91,
H 5.67, N 15.48, S 11.75. C15H15N3S
Calculated, %: C 66.88, H 5.61,
N 15.60, S 11.90.
The purity of the
synthesized 2,6-bis(phenyl)hexahydro-1,3,5-triazine-4-tion is indicated with
the method of thin - layer chromatography. Eluent is the mixture of isopropyl
alcohol and hexane (3:1 ratio). The presence of the blot has explained with
iodine smoke.
IR spectrums have painted
in Specord – 75 spectrometer with suspension prepared in Vaseline oil.
NMR 1H and 13C
spectrums are extracted in Bruker (300 Mhs) apparatus.
2,6-bis(phenyl)hexahydro-1,3,5-triazine-4-tion`s 1H
NMR spectrum (δ, m.h.): 5.67 (δ, 2H,
2CH); 7.30-7.48 (m, 10H, 2Ar); 10.49 (δ, 2H, 2NH); 11.41 (δ, 1H, NH).
13C NMR spectrum (δ,
m.h.): 65.53 (2CH), 126.33 (4CH-Ar), 129.51 (4CH-Ar), 129.52 (2CH-Ar), 140.01
(2C-Ar), 173.18 (C=S).
RESULT AND DISCUSSION
Taken into consideration information showing above, by
three – component condensation it has been synthesized
hexahydro-1,3,5-triazine-4-tion and optimal condition for increasing the yield
was also found. It is necessary to show that three – component cyclization
reactions are complex reactions. It is normal to obtaining the accessory
substances also with the basic substances. In obtaining reaction of
hexahydro-1,3,5-triazine-4-tions by receiving accessory substances as azomarks
result in reducing the yield. To increase the yield it is necessary to found
optimal conditions. Thus, by using the better optimal digs was synthesized
2,6-bis(phenyl)hexahydro-1,3,5-triazine-4-tion with 65% yield.
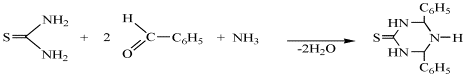
Three – component condensation reaction with thiourea,
benzaldehyde and 33.5% water solution of ammonia was investigated to study the
direction of the reaction by the scheme showed above and to increasing the
yield of necessary substances.
Therefore analogical reactions were conducted and the fewer yields was
got. This fact shows that three – component condensation on the basis of
thioures, aliphatic or aromatic aldehydes and primary amines needs carrying out
by different ways.
REFERENCES
1. Pesticides. Chemistry, technology and apply. Ì.: 1987, 639-661.
2. Common organic chemistry. Trans. From eng. Ì.: 1985, 8,
185-194.
3. Hearn, M. J.; Levy, F. Organic
preparation and procedures int. 1984, 16, 3-4, 199-277.
4. Chemical Encyclopedia. Pub-on “
Great Russian Encyclopedia” Moscow. 1995, 1248-1251.