Protsenko V.S., Vasil'eva E.A., Smenova I.V.,
Danilov F.I.
Ukrainian State University of Chemical Technology,
Dnepropetrovsk, Ukraine
Nano-structured Fe/ZrO2 composite
coatings electrodeposited from methanesulfonate electrolyte
The electrodeposition of iron, its alloys and
composites has been widely used for different engineering applications [1–4]. Iron-containing
deposits may be obtained from acidic sulfate, chloride, fluoroborate and
sulfamate Fe(II) electrolytes. Aqueous Fe(II) baths on the basis of methanesulfonic
acid (MSA) seem to be an attractive and perspective alternative to common iron
electroplating baths [5, 6] since MSA is considered as a "green acid"
due to its environmental advantages [7–10]. The performance parameters
of iron-based electrodeposits can be improved by the incorporation of dispersed
particles into metallic matrix [11, 12]. In the present work, the
electrodeposition of composite Fe/ZrO2 coatings has been
investigated using a methanesulfonate electrolyte.
Iron was
deposited from the bath containing 1.25 M Fe(CH3SO3)2.
The bath temperature was 298 K and the pH
value was 1.3. Doped zirconia nanopowder
ZrO2 + 3 mol% Y2O3 was applied for
obtaining composite iron-zirconia coatings. Mono-dispersed
nanopowders of stabilized zirconia contained particles with a prescribed size
of about 18 nm. Doping by 3 mol% Y2O3
was used for the purpose of stabilizing the tetragonal phase of zirconia.
It
should be observed that the iron coatings electrodeposited from the
methanesulfonate bath have a nano-crystalline structure, an average grain size
being about 40 nm.
The
content of ZrO2 particles in the composite coatings increases with
an increase in the suspension concentration and decreases somewhat with an
increase in the cathodic current density (Figure 1). On the basis of analyzing the
experimental data, we have stated that
the mechanism of composite coatings formation in this work satisfactorily obeys
a kinetic model proposed by Guglielmi [13].
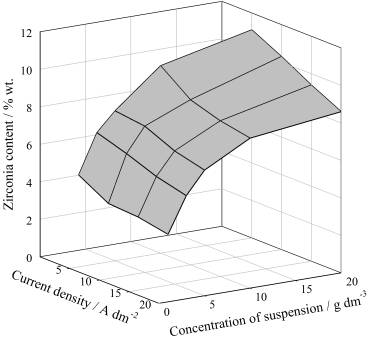
Figure 1. Effect of current
density and zirconia concentration in the bath on the ZrO2 content
in the composite coatings
Figure
2 demonstrates that the introduction of
yttria-stabilized ZrO2 particles into the iron
matrix leads to an increase in the microhardness of the coatings. A growth in
suspension concentration results in an increase in the deposits hardness.
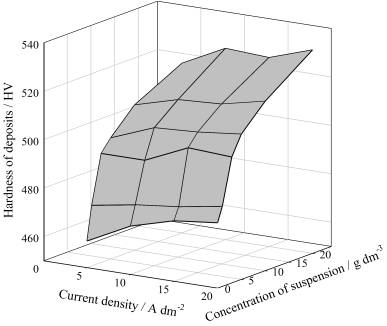
Figure 2. Effect of current density and zirconia concentration in the
bath on the deposits microhardness
We suppose that the enhanced hardness of composite Fe/ZrO2
coatings in comparison with pure iron is due to the dispersion strengthening
(i.e. strengthening effect by the
Orowan mechanism) [14, 15]. A dispersion strengthened composite is
characterized by a dispersion of fine particles which impede the motion of the
dislocations in metallic matrix resulting in an increase in material hardness.
References
1. S.L. Díaz, J.A. Calderón, O.E.
Barcia, O.R. Mattos, Electrochim. Acta 53 (2008) 7426.
2. N. Miyamoto, K. Yoshida, M. Matsuoka, J. Tamaki, J. Electrochem.
Soc. 151 (2004) C645.
3. F.I. Danilov, V.S. Protsenko, A.V. Ubiikon', Russ. J. Electrochem. 41 (2005) 1282.
4. P. Fu, C. Zhao, H. Tian, Adv. Mater. Res. 183-185
(2011) 1539.
5. E.D. Pleshka, Surf. Eng. Appl. Electrochem. 44
(2008) 92.
6. E.D. Pleshka, Surf. Eng.
Appl. Electrochem. 44 (2008) 264.
7. M.D. Gernon, M. Wu,
T. Buszta, P. Janney, Green Chem. 1 (1999) 127.
8. F.I. Danilov, T.E. Butyrina, V.S. Protsenko, E.A.
Vasil'eva, Russ. J. Appl. Chem. 83 (2010) 752.
9. F.I. Danilov, E.A. Vasil'eva, T.E. Butyrina, V.S. Protsenko, Prot. Met.
Phys. Chem. Surf. 46 (2010) 697.
10. F.I. Danilov, V.S. Protsenko, E.A. Vasil'eva, O.S. Kabat, Trans.
Inst. Metal Finish. 89 (2011) 151.
11. C.T.J. Low, R.G.A. Wills, F.C. Walsh, Surf.
Coat. Technol. 201 (2006) 371.
12. P. Zhou, Y. Zhong, H. Wang,
L. Fan, L. Dong, F. Li, Q. Long, T. Zheng, Electrochim. Acta 111 (2013) 126.
13. N. Guglielmi, J. Electrochem. Soc. 119 (1972) 1009.
14. W. Wang, F.-Y. Hou, H. Wang, H.-T. Guo, Scripta
Mater. 53 (2005) 613.
15. S. Mohajeri, A. Dolati, Mater. Chem. Phys.
129 (2011) 746.