Korniyevsky Yu.I.,
Gudzenko A.V., Korniyevska V.G.,Panchenko
S.V.
THE DEFINITION OF THE CONTENT OF PHENOLIC COMPOUNDS OF
SPECIES OF THE GENUS OF VALERIAN
Zaporozhye State Medical University
State laboratory of the quality control of medicines
SI"Institute of Pharmacology and Toxicology of the NAMS of Ukraine»
Environmental
problems in Ukraine, as well as all over the world, have initiated a new search
for alternative methods of treatment, including phytotherapy. Real
effectiveness and high safety level are making phytotherapy an essential means
for a prolonged treatment of chronic diseases. It is an urgent problem to
conduct a phytochemical research of species of the genus of Valeriana L., which
were obtained from biologically active substances and introduced into practice as an additional
sources of medicinal plant raw
material[1-8].
The aim of the work is
to research the phenolic compounds in the raw materials of different species of
the genus of valerian.
Materials and methods of research. Samples of leaves, roots, inflorescences of Valeriana collina Wallr were harvested in Zakarpatska area,
Mukachevsk district, v. Synyak. Samples of roots of V. grosshemii Worosch were obtained from the research area of the
village of Tomakivka, Dnipropetrovsk region. The aerial part and roots of V. stolonifera Czern were taken from
Zaporozhye area, beam Kantserivska. Samples of roots and inflorescences of V. exaltata Mikan were received rom c.
Zaporizhzhya from the Right Bank district of the Dniper river.
There
was performed anextraction ofbiologically active substances from the researched
raw material. 0,5 g of minced raw material (for mono materials) were placed
into a conical flask of 100 ml, equipped with refluxcondenser. 25 ml of mixture
of the following composition: 96% ethyl
alcohol: water: 25% hydrochloric acid (25: 20: 5) was added there.The
flask was boiled for 90 minutes in a water heater. The samples were cooled to
room temperature (200C) and after that the extract was filtrated to a volumetric flask with
the capacity of 25 ml.
The
chromatographic study of the researched samples was performed on liquid
chromatograph equipped with diode array detector Shimadzu HPLC-system, ser.20.
The
study was done under following conditions: the column Phenomenex Luna C18 (2)
measured 250 mm x 4,6 mm. Theparticle
size was 5 micron. The temperature of the column was 350C. The detection wavelength composed 330 nm.The mobile
phase flow rate made 1 ml per minute. The volume of introduced sample was - 5 mcL.
The
mobile phasecomposed:
|
Time
of Chromatography (min) |
Eluent А, % |
Eluent B, % |
|
0–5 |
95 |
5 |
|
5–35 |
95 → 75 |
5 → 25 |
|
35–40 |
75 |
25 |
|
40–60 |
75 → 50 |
25 → 50 |
|
60–65 |
50 → 20 |
50 → 80 |
|
65–70 |
20 |
80 |
|
70–85 |
95 |
5 |
Eluent A was 0.1% solution of trifluoroacetic
acid in water.
Eluent B was 0.1% solution of trifluoroacetic acid
in acetonitrile.
The
identity of the components was performed by retention time matching of UV-
spectra to the range of standard substances spectra.
The following conditions were used in the study without hydrolysis.
Extraction
ofbiologically active substances in the researched raw material was
performed. 0,5 g (the exact sample) of
minced raw materialwas introduced into a conical flask of 100 ml, equipped with
reflux condenser.25 ml of 50% ethyl
alcohol was added there. The flask was boiled for 45 minutes in a water
heater. After that the extractwas cooled to room temperature was filtrated
through the filter of "red tape" to a volumetric flask with the
capacity of 25 ml.The extractwasbroughtup to the volume of 25 mlby50% ethyl alcohol. The chromatographic
study of the researched samples was performed on liquid chromatograph equipped
with diode array detector Shimadzu HPLC-system, ser.20.
The
study was done under following conditions: the column Phenomenex Luna C18 (2)
measured 250 mm x 4,6 mm. The particle size was 5 micron. The temperature of
the column was 350C.
The detection wavelength composed 330 nm.The mobile phase flow rate made 1 ml
per minute. The volume of introduced sample was - 5 mcL.
The
mobile phasecomposed:
|
Time
of Chromatography (min) |
Eluent А, % |
EluentB, % |
|
0–5 |
95 |
5 |
|
5–35 |
95 → 75 |
5 → 25 |
|
35–40 |
75 |
25 |
|
40–60 |
75 → 50 |
25 → 50 |
|
60–65 |
50 → 20 |
50 → 80 |
|
65–70 |
20 |
80 |
|
70–85 |
95 |
5 |
Eluent A was 0.1% solution of
trifluoroacetic acid in water.
Eluent B was 0.1% solution of trifluoroacetic
acid in acetonitrile.
The identity of
the components was performed by retention time and matching ofUV- spectra tothe
standards of the substances.
Results and discussion.
The comparative quantitative content of phenolic compounds in different organs
of valerian is presented in Table 1,2. The chromatograms of phenolic compounds
are shown in Fig. 1-16.
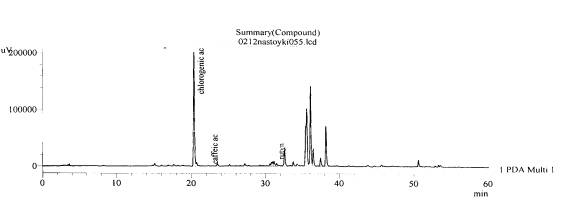
Fig. 1.The chromatogram of raw
material Valeriana collinaWallr Zakarpatska
area, Mukachevsk district, v. Synyak– leaves (without hydrolysis)
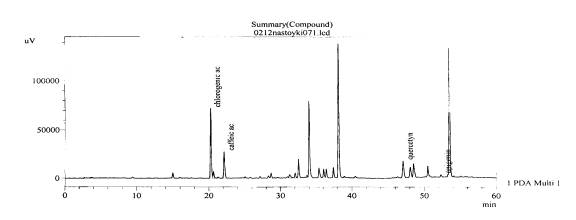
Fig. 2.The chromatogram of raw material Valeriana collina Wallr Zakarpatska area, Mukachevsk district, v. Synyak – leaves (with hydrolysis)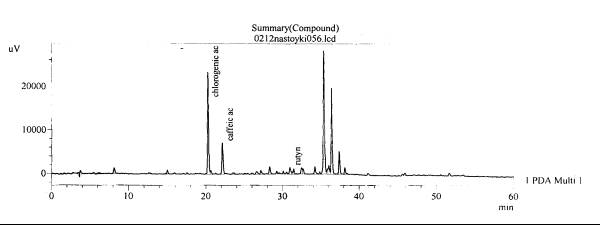
Fig. 3.The chromatogram of raw
material Valeriana collina Wallr Zakarpatska
area, Mukachevsk district, v. Synyak–roots (without hydrolysis)
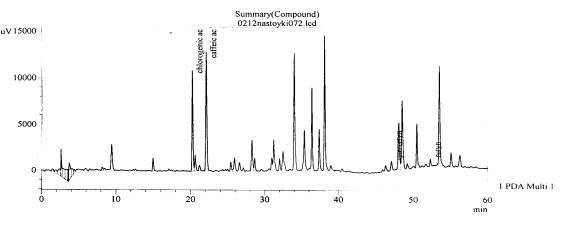
Fig. 4.The chromatogram of raw
material Valeriana collina Wallr Zakarpatska
area, Mukachevsk district, v. Synyak–roots (with hydrolysis)
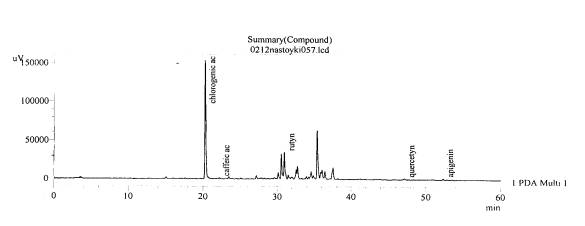
Fig. 5.The chromatogram of raw material
Valeriana collina Wallr Zakarpatska
area, Mukachevsk district, v. Synyak–inflorescences (without hydrolysis)
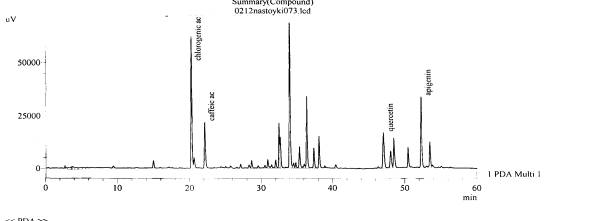
Fig. 6.The chromatogram of raw
material Valeriana collina Wallr Zakarpatska
area, Mukachevsk district, v. Synyak–inflorescences(with hydrolysis)
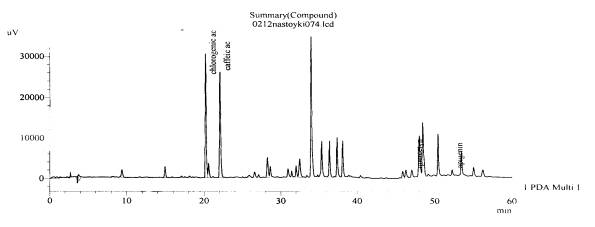
Fig. 7.The chromatogram of raw
material V. grosshemii Worosch the
research area of the village of Tomakivka, Dnipropetrovsk region–roots (with
hydrolysis)
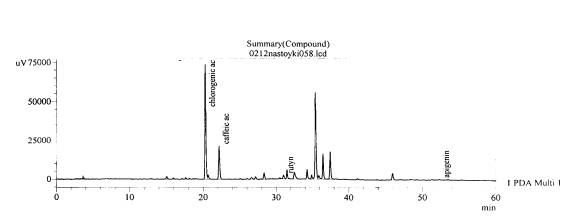
Fig. 8.The chromatogram of raw
material V. grosshemii Worosch the
research area of the village of Tomakivka, Dnipropetrovsk region –roots
(without hydrolysis)
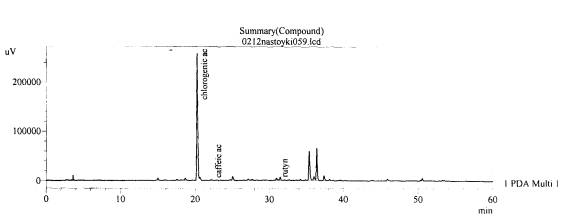
Fig. 9.The chromatogram of raw
material V. stolonifera Czern Zaporozhye
area, beam Kantserivska–aerial part(without hydrolysis)
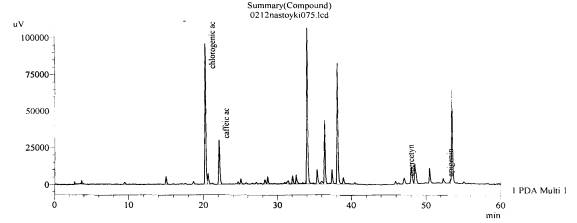
Fig. 10.The chromatogram of raw
material V. stolonifera Czern Zaporozhye
area, beam Kantserivska–aerial part(with hydrolysis)
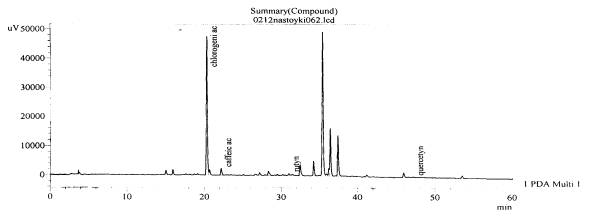
Fig. 11.The chromatogram of raw
material V. stolonifera Czern Zaporozhye
area, beam Kantserivska– roots (without hydrolysis)
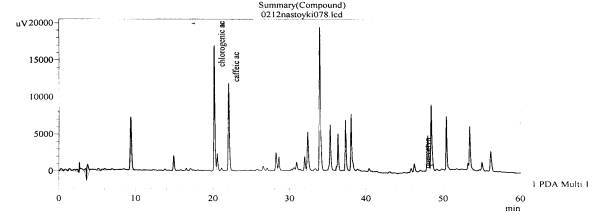
Fig. 12.The chromatogram of raw
material V. stolonifera CzernZ
aporozhye area, beam Kantserivska–
roots(with hydrolysis)
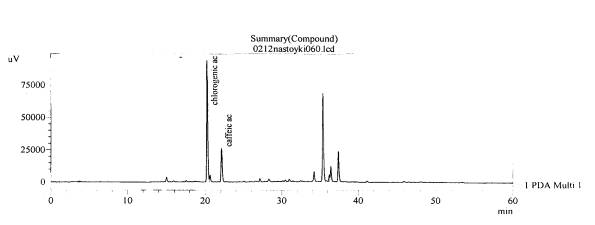
Figure 13.The chromatogram of raw
material V. exaltata Mikanc.
Zaporizhzhya at the Right Bank district of the Dniper river– roots(without
hydrolysis)
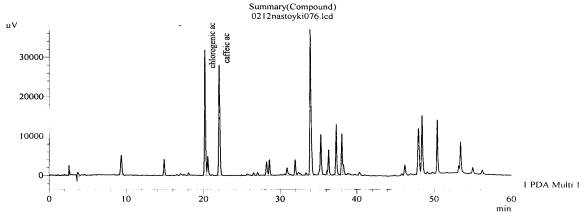
Figure 14.The chromatogram of raw
material V. exaltata Mikanc.
Zaporizhzhya at the Right Bank district of the Dniper river – roots(with
hydrolysis)
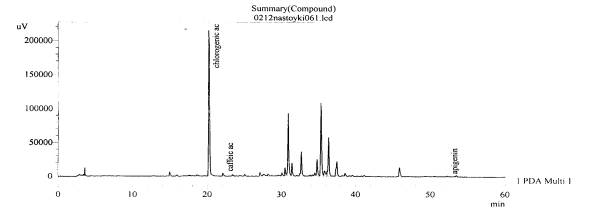
Figure 15.The chromatogram of raw
material V. exaltata Mikan c.
Zaporizhzhya at the Right Bank district of the Dniper river–inflorescences
(without hydrolysis)
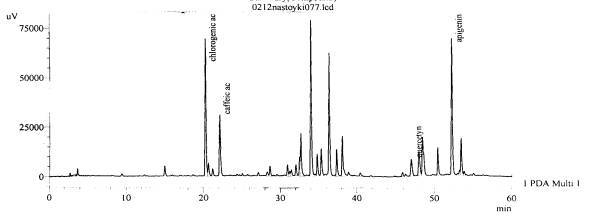
Figure 16.The chromatogram of raw
material V. exaltata Mikanc.
Zaporizhzhya at the Right Bank district of the Dniper river– inflorescences
(with hydrolysis)
Table 1
The content of flavonoids in raw materials of different species of valerian
|
Raw material Substance, % |
Rutin |
Quercetin |
Apigenin |
|||
|
Without hydrolysis |
Without hydrolysis |
With hydrolysis |
Without hydrolysis |
With hydrolysis |
||
|
V. collina |
inflore-scences |
0,294 |
0,01 |
0,1222 |
0,0058 |
0,129 |
|
leaves |
0,0747 |
|
0,123 |
|
0,0103 |
|
|
roots |
0,01528 |
|
0,00711 |
|
0,00286 |
|
|
V. grosshemii |
roots |
0,0272 |
|
0,0122 |
0,001157 |
0,00575 |
|
V. stolonifera |
aerial part |
0,0217 |
|
0,030 |
|
0,0139 |
|
roots |
0,00589 |
0,00078 |
0,00121 |
|
|
|
|
V. exaltata |
inflore-scences |
|
|
0,06115 |
0,00399 |
0,2643 |
The
data presented in Table 1 proved that the inflorescences of Valeriana collina Wallr contained the
greatest quantity of flavonoids: 0,294%ofrutin, 0,01% and 0,1222%of quercetin,
0,129% and 0,0058%ofapigenin. In the leaves of the same species of valerianwere
found: 0,0747% of rutin, 0,123%of
quercetin and 0,0103% of apigenin. Its root included0,01528% of rutin, 0,00711%
of quercetin and 0,00286% of apigenin. In the root of V. grosshemii Worosch were found 0,0272% of rutin, 0,0122% of
quercetin, 0,00575%and 0,001157% of apigenin. In the aerial part of V. stolonifera Czern valerian were
identified 0,0217% of rutin, 0,030% of quercetin, 0,0139% of apigenin. In the
rootsof thesespecies were found 0,00589%and 0, 00078% of rutin, 0,00121% of
quercetin. In inflorescences of V.
exaltata Mikan were discovered 0,06115% of
quercetin,0,00399% and 0,2643% of apigenin.
Table 2
Thecontentofthe phenolic acidsin raw materials of
different species of valerian
|
Raw material Substance, % |
Chlorogenic acid |
Caffeic
acid |
|||
|
Without hydrolysis |
With hydrolysis |
Without hydrolysis |
With hydrolysis |
||
|
V. collina |
inflore- scences |
0,5106 |
0,1997 |
0,00302 |
0,05078 |
|
leaves |
0,669 |
0,2271 |
0,00189 |
0,0635 |
|
|
roots |
0,0699 |
0,042 |
0,01705 |
0,0296 |
|
|
V. grosshemii |
roots |
0,2411 |
0,0965 |
0,050 |
0,0609 |
|
V. stolonifera |
aerial part |
0,8546 |
0,306 |
0,00377 |
0,0701 |
|
roots |
0,1534 |
0,0545 |
0,0054 |
0,0272 |
|
|
V. exaltata |
inflore- scences |
0,6959 |
0,2203 |
0,0092 |
0,0718 |
|
roots |
0,30209 |
0,101 |
0,0611 |
0,0653 |
|
The
data presented in Table 2 demonstrated
the content of phenolic acids in the raw material. The inflorescences of Valeriana collina Wallr counted 0,5106%
and 0,1997% of chlorogenic acid, 0,00302% and 0,05078% of caffeic acid. In the
leaves of Valeriana collina Wallr were
founded0,669% and 0,2271% of chlorogenic acid, 0,00189% and 0,0635% of caffeic
acid. The roots contained 0,0699% and 0,042% of chlorogenic acid, 0,01705% and
0,0296% of caffeic acid. In the rootsof V.
grosshemii Worosch were accounted0,2411% and 0,0965% of chlorogenic acid,
0,050% and 0,0609% of caffeic acid. 0,8546% and 0,306% of chlorogenic acid,
0,00377% and 0,0701% of caffeic acid
were revealed in the aerial part of V.
stolonifera Czern. 0,1534% and 0,0545% of chlorogenic acid, 0,005 and
0,0272 of caffeic acid were identified in the roots of V. stolonifera Czern. In inflorescences of V. exaltata Mikan were detected 0,6959% and 0,2203% of chlorogenic
acid, 0,0092 and 0,0718 of caffeic acid. Caffeic acid composed 0,0611%
and 0,0653% in the roots of V.
exaltata Mikan.
Conclusions.The
following main conclusions can be drawn from the results obtained in the
present studies. The highest content of flavonoids is found in inflorescences
of Valeriana collina Wallr, namely,
0,294% of rutin, 0,1222% of quercetin
and 0,129% of apigenin. The leaves of Valeriana
collina Wallr contain a large number of quercetin about 0,123%. The greatestamount
of chlorogenic acid is traced in the aerial part of V. stolonifera Czern and composes 0,8546%. Somewhat smaller content
is in leaves (0,669%) and inflorescences (0,5106%) of Valeriana collina Wallr,
and in inflorescences (0,6959%)V.
exaltata Mikan. The largest quantity of coffee acids is identified in the
roots (0,0611%) of V. exaltata Mikan
and in the roots (0,050%) V. grosshemii
Worosch.
The
aerial and belowground parts of different species of valerianwereanalyzed. The
results of the study proved that flavonoids, especially rutin, was found in the
aerial part of the plantin greater amounts.Among the studied phenolic acids the
biggest amount of chlorogenic acid is contained in the belowground samples.
REFERENCES
1.
Валерианотерапия
нервно-психических болезней / Н.С. Фурса, Е.А.Григорьева, В.Г. Корниевская и
др.- Запорожье : "ИВЦ с/х", 2000.- 348 с. 2.
2. 2.Валериана в
фитотерапии / Н.С. Фурса, А.А. Зотов, СЕ. Дмитрук, С.Н.Фурса.// Томск:
НТЛ 1998.- 212 с.
2.
Державна
Фармакопея України / Державне підприємство «Науково-експертний
фармакопейний центр». -1-е
вид. - Харків: РІРЕГ, 2001. - С 556 с, Доповнення 1.
- Харків: РІРЕГ. - 2004. - 520с, Доповнення 2. - Харків: РІРЕГ. -
2008. - 608с.
3.
Корнієвська В.Г. Порівняльне фармакогностичне дослідження валеріани пагоносної та валеріани
високої: автореф. дис... .канд. фармац. наук.-Львів, 2002.- 20 с.
4.
Корнієвська В.Г., Фурса М.С. Порівняльне вивчення вмісту флавоноїдів і поліфенольних сполук валеріани
пагононосної та валеріани високої протягом доби // Фізіологічно активні
речовини. - 2001. - Т. 31, № 1. – С.82 - 86.
5.
Литвиненко
В.И.
Химия природных флавоноидов и создание препаратовпри комплексной переработке
растительного сырья:дис. в форме науч.доклададок.хим. наук.- X.,
1990.- 79 с.
6.
Монографія «Валеріана лікарська» / Корнієвський
Ю.І.,Корнієвська В.Г.,Панченко С.В.,Богуславська Н.Ю. //
Запоріжжя:ЗДМУ,2014.-501с.
7.
Панченко С.В.
Порівняльне фармакогностичне дослідження валеріани Гросгейма з іншими видами
роду валеріана: автореф. дис…канд..фармац. наук.-Запоріжжя, 2014.- 24 с.
8.
Wang, Peng-Cheng et al. / Phenolic compounds from the roots of Valerianaofficinalisvarlatifolia.
// J. Braz. Chem. Soc. – Sept 2013, vol.24, no.9,
p.1544-1548.
List of Authors:
KorniyevskyYu.I. - Candidate of Pharmaceutical
Sciences (PhD), Associate Professorof the Department of Pharmacognosy, Pharmacology and Botany of
ZSMU;
Gudzenko A.V. - Doctor of
Pharmaceutical Sciences (Dr), researcher of the State laboratory of the quality
control of medicines SI "Institute of Pharmacology and Toxicology of NAMSof Ukraine ";
KorniyevskaV.G.- Candidate of
Pharmaceutical Sciences (PhD), Associate Professor of the Department of
Pharmacognosy, Pharmacology and Botany of ZSMU;
Panchenko S.V. - Candidate of
Pharmaceutical Sciences (PhD), the assistant of the Department of Pharmacognosy, Pharmacology and Botany of
ZSMU.
Address for correspondence:
Korniyevsky Yuri Ivanovich, 69032 Zaporozhye, drivewayDruzhniy, house 9A, fl.
22,email address: kornievsk@gmail.com