Êhaymuldinova Altyngul Kumashevna,
Bulasheva Aigul Imangalievna, Eszhanov Galihan Serdalinovich
Kokshetau State Universitynamed after Sh.Ualihanov,
str. Kuanyshev 170 (a), Kokshetau 000020, Akmola region, The Republic of
Kazakhstan
Changes in the quality of the fat phase
biocomplex to increase the protective functions of the body, depending on the
length of the dispersion
Keywords: dispersing, linoleic,
linolenic and arachidonic pentatenovaya, biocomplex oxidation reagent, acid,
peroxide number
Summary
Scientifically based method of lightening the
whole blood of horses without the use of chemicals by dispersing its
biocomposition using physical methods of treatment, with a sufficiently high
biological value and using it in meat production.
The optimal technological
parameters of production biocomplex (temperature insertion of components,
selection and order of mixing, the dispersion (maximum dispersion and stability
biocomposition observed at τ = 7 min., Average adipose particles = 1.96 mm), the lack of
hydrolytic and oxidative processes in fat biocomplex (within 6-10 minutes of
acid and peroxide are not changed and remain within 1.10-1.15 and 0,021-0,023
level control; PUFAs are not destroyed).
1 Changes in the content of
polyunsaturated adipose acids
To address the question of
whether to use mechanical processing in the manufacture of biocomplex decisive
factor is the quality of the product. Therefore, it became necessary to study
changes in the adipose phase biocomplex depending on the length of dispersion.
As a control experiment, adopted pork meat.
In addition to the peroxide and acid number of
adipose quality can be characterized by changes in the content of
polyunsaturated adipose acids such as linoleic, linolenic and arachidonic, are
vital and least stable at various physical and chemical influences.
Data on the change in the content of conjugated
compounds and peroxide value and acid number of adipose particles, depending on the length of the dispersion are given in Tables 1 and
2.
Table -1 Change of acid and peroxide numbers of
adipose particles in the process of
dispersing
|
Indicators |
¹ experience |
Thetreatmenttime, min |
|||||
|
Contact role |
3 |
5 |
7 |
10 |
12 |
||
|
Àcidnumber |
1 |
1,15 |
1,15 |
1,18 |
1,18 |
1,17 |
1,19 |
|
2 |
1,10 |
1,10 |
1,12 |
1,12 |
1,10 |
1,10 |
|
|
|
3 |
1,13 |
1,13 |
1,12 |
1,12 |
1,12 |
1,12 |
|
Ì |
|
1,13 |
1,13 |
1,14 |
1,14 |
1,13 |
1,14 |
|
± m |
|
0,015 |
0,015 |
0,020 |
0,020 |
0,024 |
0,027 |
|
Peroxidenumber |
1 |
0,021 |
0,021 |
0,021 |
0,020 |
0,020 |
0,020 |
|
2 |
0,020 |
0,020 |
0,019 |
0,019 |
0,019 |
0,019 |
|
|
3 |
0,023 |
0,023 |
0,022 |
0,022 |
0,023 |
0,013 |
|
|
Ì |
|
0,021 |
0,021 |
0,021 |
0,020 |
0,021 |
0,018 |
|
± m |
|
0,0009 |
0,0009 |
0,0009 |
0,0009 |
0,0009 |
0,0009 |
Table 2 - Changes in the
content of compounds with conjugated double ties in the process of dispersing
|
PairedConnection |
¹ experience |
Thedispersiontime, min. |
|||||
|
Contact role |
3 |
5 |
7 |
10 |
12 |
||
|
ÕÑ |
1 |
0,810 |
0,805 |
0,810 |
0,810 |
0,810 |
0,810 |
|
2 |
0,782 |
0,780 |
0,783 |
0,782 |
0,783 |
0,783 |
|
|
3 |
0,811 |
0,810 |
0,812 |
0,810 |
0,810 |
0,811 |
|
|
Ì |
|
0,801 |
0,798 |
0,802 |
0,801 |
0,801 |
0,801 |
|
± m |
|
0,0095 |
0,0093 |
0,0093 |
0,0093 |
0,0090 |
0,0092 |
|
ÓÑ |
|
- |
- |
- |
- |
- |
- |
|
ZC |
|
- |
- |
- |
- |
- |
- |
|
UC |
1 |
0,0163 |
0,0165 |
0,0163 |
0,0165 |
0,0166 |
0,0166 |
|
2 |
0,0141 |
0,0142 |
0,0141 |
0,0140 |
0,0141 |
0,0141 |
|
|
3 |
0,0156 |
0,0155 |
0,0156 |
0,0155 |
0,0156 |
0,0156 |
|
|
Ì |
|
0,0153 |
0,0154 |
0,0153 |
0,0153 |
0,0154 |
0,0154 |
|
± m |
|
0,00065 |
0,00067 |
0,00065 |
0,00073 |
0,00073 |
0,00073 |
As seen from the results shown in Tables 1-2,
the amount of diene, triene, tetraene and pentaenoate with conjugated bonds
does not change the duration of machining. Thus, we can say that the
polyunsaturated fatty acids: linoleic, linolenic and pentanoicarachidonic in
the formation of the emulsion is not broken, therefore the quality and
nutritional value of the fat phase of the duration of mechanical stress is not
reduced.
Acid and peroxide values of adipose particles
in the dispersion process are not changed and remain at the level of control.
2 The study of lipid peroxidation depending on the
length of dispersion
It is known that in the
process of dispersing adipose particles with the help of mechanical impact possible physical
and chemical changes of the object, manifested in destructive rearrangements
and lipid peroxidation.
The purpose of this phase of
the study to explore the change in the rate of accumulation of hydroperoxides
are the primary products of adipose oxidation, chemiluminescence method. This
method and the relative simplicity allows to fix the presence of hydroperoxide
concentration of 10-7-10-8 mol / liter, whereas the sensitivity of a standard
method of 10-4-10-6 mol / liter. The rate of lipid peroxidation is determined
by many factors: the composition of the mixture, the presence of factors that
inhibit and catalyzing process speed, temperature, oxygen availability, and
other antioxidants. Injection biocomplex in the blood may have combined value
to adipose oxidation as it contains substances which are may both enhance and
inhibit lipid peroxidation.
Injection of the biocomplex broth,
melange and other additives, having a strong emulsifying action, can slow down
the process of lipid peroxidation. In connection with these studies have established
that the influence of mode of mechanical action on the rate of peroxidation of
lipids of the adipose phase biocomplex. For this were studied the rate of lipid
peroxidation and total antioxidant activity. Lipid peroxidation was studied as
a function of time for processing the samples biocomplex. Samples were
processed with a homogeniser for 1, 3, 5, 7, 9, 12 and 15 minutes. Comply with
the same composition, temperature biocomplex, as well as the intensity of the
elastic vibrations in the processing of a homogenizer.
The data presented in Figure 1
demonstrate that increasing the duration of treatment, an increase of the
induction period, designated τ-period, indicating
that the increased antioxidant activity of the lipid. The increase in τ-period is biphasic: the first stage (up to 3
minutes), there is an increase it, and then a slight decrease (7 minutes). Such
a dependence of τ-period of the
duration of mechanical treatment may be a consequence of the opposite effects
on lipid peroxidation biocomplex components: blood and masses of
"Karty." On the one hand, the blood pigments - hemoglobin and its
derivatives containing heme iron are potent catalysts for the oxidation of
lipids; on the other hand - the presence in the membranes of red blood cells
tocopherol, having the properties of a strong antioxidant, inhibits lipid
peroxidation, formation of lepidopterology, lepidorhinusfraction. Formation and
lepidopterology
andlepidorhinusfractions ensures lightening whole blood. Presence of
biocomplex broth melange, sodium ascorbate and other additions leads to the
inhibition of lipid peroxidation, as it has a high antioxidant activity.
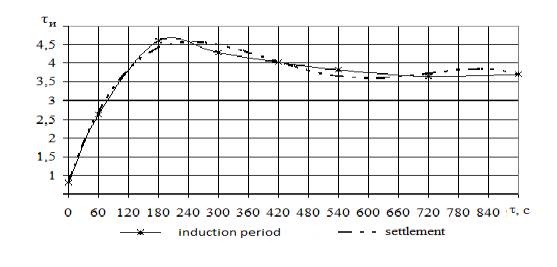
τè = 8·10-11
τ4 + 2·10-7 τ3 – 0,0001τ2 +0,0389 τ+ 0,8145 R2
= 0,98
Figure 1 - Change
in the injection period biocomplex depending on the length of machining
The antioxidant capacity of
the above additives due to their high emulsifying properties, providing a high
degree of dispersion of the fat phase biocomplex and building strong adsorption
lipodepsipeptide, lepidodendraceshells on the surface of the adipose particles,
preventing their coalescence. The presence of such layers not only provides
stability biocomplex, but also an important factor in inhibition of oxidative
processes in emulsified adipose particles. Furthermore, the antioxidant
properties of the broth and melange explained, firstly, their ability to bind
to divalent iron ions to form inactive in respect of the catalytic components;
Secondly, due to the presence within their structure of SH - OH groups can have
high antioxidant activity against lipid. This position is supported by the data
to determine the rate of lipid peroxidation by a TAC biocomplex.
Figure 2 shows that with
increasing duration of mechanical treatment in the early stages there is some
increase and then a sharp decline in the accumulation of lipid peroxidation
products. This can be explained by the fact that the first is a free
non-emulsified adipose oxidation, the surface of which there is no layer of stabilizer.
With the increase in the dispersion stability and dispersion of biocomplex
increases, the amount of stabilized adipose phase increases, formed a strong
adsorption lipocarotene, leptophlebia, lepidosirenidaeshell that prevents
oxidation of lipids.![]()
ÒÁÊ= 3·10-11 τ4 + 7·10-8 τ3 - 5·10-5 τ2 + 0,013 τ +1,0554 R2=0,97
Figure 2 - Changes in titratable
acidity biocomplex protein depending on the length of machining
This result is confirmed by
the previously obtained data indicating that the most stable biocomplex with
minimum particle size of the adipose phase obtained by the processing time 7
min.
Change in the amplitude quick
flash chemiluminescenceaccording to the length of dispersion are shown in
Figure 3 Variation of the amplitude quick flash confirms the dependence of
lipid peroxidation on the degree of stability and dispersion biocomplex,
namely: first, the amplitude of the flash increases as the rate of formation of
hydroperoxides is higher than the rate of their destruction. However, with
increasing duration of treatment, behind a growing stability biocomplex, the
rate of formation of hydroperoxides decreases and reaches a minimum value at 7
min. processing. After a 7-minute treatment biocomplex stability is somewhat
reduced, which affects the rate of increase in lipid peroxidation, as evidenced
by the amplitude of the fast flash at 9, 12 and so on. G. Minute processing.
The increase in the rate of lipid peroxidation
in the initial period is due to the oxidation of free adipose, not more
involved in education biocomplex. In the second period the increase in the
dispersion leads to a complete structure formation system (maximum dispersion),
which is accompanied by the formation of shells around the adipose particles
and involvement in the regulation of lipid peroxidation antioxidants blood.
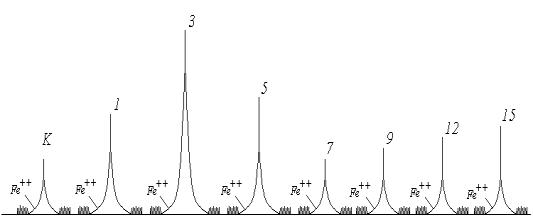
Figure 3 -
Variation of the amplitude quick flash chemiluminescence according to the
length of dispersion
Further increase in the length
of the machining reduces the dispersibility, a certain increase in the content
of free adipose particles and as a consequence, increase the rate of lipid
peroxidation and decrease of antioxidant activity.
We can say that the presence
in the mass of biocomplex "Karty" which is a structural antioxidant,
slows the formation of hydroperoxides, thus the quality of the adipose
particles phase in the dispersion process is not reduced.
Studies performed to study the
changes in the adipose particles phase in the manufacturing process biocomplex
revealed that the acid value and peroxide properties, as well as compounds with
conjugated double bonds when dispersed on the cutter during 1, 3, 5, 7 minutes
remained at the control sample. This indicates that no fine grinding increases
the rate of oxidation of adipose particles and destroys vital polyunsaturated
adipose acids such as linoleic, linolenic, arachidonic and eicosapentaenoate,
i.e. nutritional value of adipose particles in the dispersion process is not
changed.
Study of changes in the rate
of formation of hydroperoxides - primary products of fat oxidation -
chemiluminescence method showed that a slight increase in the rate of formation
of hydroperoxides at the beginning of the dispersion process due to oxidation
of emulsified adipose particles. With increasing dispersionbiocomplex increases
the amount of emulsified adipose particles and reduces the amount of
hydroperoxides. By 7 minutes dispersion rate of formation of hydroperoxides
reaches its minimum value. A slight increase in the amount ofhydroperoxides by
dispersing more than 7 minutes explained destruction biocomplex with the
release of free adiposeparticles.
This position is also
supported by the value of antioxidant activity, which is characterized by the
duration of the induction period and is in inverse proportion to the
relationship to lipid peroxidation. Changing the AOA and LPO depends on the
rate of formation of the fine system, which in turn depends on the emulsifying
ability of an emulsifier, such as broth, melange and nitrite.
Such a move depending on the rate of
increase of the duration of hydroperoxides dispersion due to the presence in
the composition of the above additives biocomplex with high emulsifying
capacity, which provides a strong adsorption membranes around the fat
particles, preventing the coalescence of fat particles as well as the contact
of the fat phase with oxygen in an aqueous medium.
Broth, egg and other additives
have been incorporated into the biocomplexare structural antioxidants,
inhibiting the rate of rise of hydroperoxides during the dispersion process.
Thus, the quality of the
adipose phase in the dispersion process using mechanical impact is not reduced,
ensures the formation of new biological complexes that increase the protective
functions of the organism.
Ñonclusion. Plural conclusions. Thus, from the above
literature data it can be concluded that in creating products for dietary food
the aim of achievement of their specific biological and nutritional value
through the efficient use of protein, adipose components of meat, raw milk in combination
with products of plant origin should be considered the main.
Acknowledgement: This article is based in order to create
products of high biological value. I am grateful to my research supervisor,
Doctor of Engineering, professor, corr-member of KazAAS of RK E. T. Tuleuov.
Corresponding
Author: Dr. Êhaymuldinova, Kokshetau State
University named after Sh.Ualihanov, str. Kuanysheva 170 (a),
Kokshetau 000020, Akmola region, Republic of Kazakhstan
References
1.
Sakata Ryoichi, Lee Guesong, Nagata YukihariNitrosation ofhemaglobin
from of meat products. //Nihon chikusangakkaiho. Anim.Sci and Technd-63, ¹
12.-1992. - S.1247-1252
2.
Potthast K., Europalsher 26. Fleischforscher – congress in Colorado
Springs USA // Fleischwirtschaft. – 1981. – Bd. 61, ¹ 5 - Ð. 749-772.
3.
Wiesmer – Pedersen J. Utilisation of animal blood in meat prodacts
//Food Technology. – 1979. – 33, ¹ 18 - Ð. 76-80.
4.
Boday C.R. Problems in the Development and Appllication of Rapid methods
of Assesing Protein Quality – Technology //J. Biol. Chem. – 1974. Vol. 31, ¹ 6
– Ð.73-77.
5.
Õîðîëüñêèé Â.Â.,
Ìèòàñåâà Ë.Ô., Ìàøåíöåâà Í.Ã., Áó÷èíñêàÿ À.Ã. Ìîëî÷íîêèñëûå ìèêðîîðãàíèçìû â
òåõíîëîãèè ìÿñíûõ ïðîäóêòîâ // Ìÿñíàÿ èíäóñòðèÿ. – Ì., 2006. - ¹ 5 - Ñ. 34-36.
6.
Boday C.R. Problems in the Development and Appllication of Rapid methods
of Assesing Protein Quality – Technology //J. Biol. Chem. – 1974. Vol. 31, ¹ 6
– Ð.73-77.
7.
21Brende J., Klein S. Der Einflure von Milcheiweremietsen auf das
WarberbindungesVercogen des lelsches //Vlelschesvirtschaft. 1972. – Bd. 52,3. –
Ð.339-340, Ð. 343-344.
8.
Sakata Ryoichi, Lee Guesong, Nagata YukihariNitrosationof hemaglobin from of meat products. //Nihon
chikusangakkaiho. Anim.Sci and Technd-63, ¹ 12.-1992. - S.1247-1252
9. EI Titov, Aleksakhina VA, VA Lisitsina, pollen LA,
Sershenko OI Blood sausage prophylactic administration. // Abstracts. II
International Workshop. Pyatigorsk, 1993. - C. 45-48.
10. Turkutyukov VB, Shimchik EA Blood spotted deer -
the raw material for foods for special purposes. // Abstracts. The inter.
nauch.konf. Vladivostok. 1993. - P. 56-59.
11. Antipova LV, SI Aslanov A new protein supplement
for combination products. // Meat prom. 1994, ¹ 4. - S. 23-25.
12. AV Antipov, Kulpina AL Using blood plasma for
industrial production of protein-containing animal products. // Meat Industry
2000, ¹ 8. - S. 5-12.
13. Pat. 5443852 proteinaceous food product comprising
a stabilized pigment heat-treated canned meat. / USA; appl. 04.30.93, opubl.22.08.95