Candidate Biol. Sci.
Malyarovskaya V.I.
The State Research Institution All–Russian
Scientific and Research Institute of Floriculture and Subtropical Crops of the
Russian Academy of Agricultural Sciences, c. Sochi, Russia, e-mail: lab-bfbr@vniisubtrop.ru
SOME CHARACTERISTICS OF
CULTIVATING ANTHERS FROM DIFFERENT CULTIVARS OF DIANTHUS
CARYOPHYLLUS IN VITRO
The paper presents the study results on cultivating
anthers from different cultivars of Dianthus
caryophyllus in vitro.
It is revealed that its genotype has the greatest impact on the efficiency of
anther culture in vitro. According to the research of Dianthus
caryophyllus different genotypes, cultivars with high androgenic
potential were allocated by responsiveness to in vitro conditions. These
include the following cultivars: Cristobal, Carnaval, Amapola, Salome and Lena.
It has been established that the ability to induce proliferation processes,
intensity of their transmission as well as the regeneration is dependent on the
composition and concentration of phytoregulators.
Key words: Dianthus caryophyllus, anther culture,
callus formation, embryogenesis, embryonic cell complexes, regenerated plants,
cytological control, diploid and haploid cells.
Introduction
One of the
most common ways to get doubled haploids is a method of induction of
androgenesis by cultivating the selected anthers. This method has been
successfully applied for many crops, particularly barley [1, 2], wheat and
triticale [3,4], maize [5], rapeseed, mustard [6], flax [7] and others.
However, as
we know, it is difficult to produce haploid plants applying the methods of
androgenesis because of a few growths (calluses and embryoid-like structures
(ELS)) and a low percentage of plant regeneration [8,9,10].
Numerous literature on the theoretical and
methodological aspects of cultivating anthers of different crops in vitro
indicate that genotypic dependence of haploid-productive process is the most
serious obstacle to the introduction of haploid technologies into the selection
[11,12,13]. In addition, recommendations on the use of only responsive forms in
anthers culture [14] and on the allocation of donors having high regenerative
capacity became widespread [15].
In
connection with this, the aim of the research was to study the genotypic
dependence of Dianthus caryophyllus on the ability to form androgenic
structures in anther culture in vitro.
Research technique
The
research was conducted in 1986-1990 yy. on the basis of Biotechnological and
Selection Departments in the Institute of Floriculture and Subtropical Crops.
22 different types of Dianthus caryophyllus were used as explants donors
(anthers): Lena, Arthur Sim, William Sim, Flamingo, Jaguar, Razdolnenskaya,
Plamya, Yubileinaya, Originalnaya, Palace, Cristobal, Vanessa, Le Re, Amapola,
Mayela, Carnaval, Claire Jellow, Samantha, Salome, Sandra, Elsie White Sim and
eight hybrids: ÎG-75-3, ÎG-87-1, ÎG-150-6, 3Ê-84-1, 3Ê-130-3, 5Ê-56-1, 8G-18-3,
3C-93-2.
Preparation
of plant material and its introduction to the tissue culture was carried out
with the requirements of aseptic techniques and methods of planting into medium
recommended by R.G. Butenko [16].
Before
introducing anthers into in vitro culture, 72-hour pretreatment with low
positive temperature (+5 °C) was performed. Immediately before planting the
anthers into culture medium, there was carried out a cytological control over
pollen developmental stages on temporary and pressure preparations which had
been painted with acetoorsein or acetocarmine solution, and analyzed them under
a microscope MBI-15. The anthers were isolated on a mononuclear pollen stage.
New growths obtained from anther culture (callus) were investigated, using
temporal cytological preparations painted with acetocarmine or hematoxylin, according
to standard procedures [17]. Callus cells were photographed (photomicrography)
and sketched, using a drawing apparatus (DA).
Carnation
buds of about 1,0 cm were sterilized in ethanol (96 %) for 5-7 minutes (laminar
box), then washed 2-3 times with distilled water. Anthers were then aseptically
singled out from the buds and placed into Murashige and Skoog medium (1962)
with different ratios and concentrations of growth regulators: Option 1
– kinetin – 0,1 mg/l, adenine – 0,5 mg/l; Option 2 - 6 BAP – 1 mg/l, NAA
– 0,3 mg/l IAA – 0,5 mg/l; Option 3 – adenine – 2 mg/l, NAA – 0,5 mg/l,
2,4-D – 3,0 mg/l, IAA – 0,5 mg/l; Option 4 - 6 BAP – 0,5 mg/l, zeatin –
0,1 mg/l; Option 5 – kinetin 2,0 mg/l, 2,4-D – 2,0 mg/l.
Anthers
were cultivated in a nutrient medium at a temperature of 24 °C, air relative
humidity was 65-70 %, firstly during 7 days in the dark, and subsequently in a
photoperiod of 16/8 with to 1500 lux illumination. In 1,5-2 months the anthers
with new formations (callus, embryonic cell complexes (ECC), rhizogenesis) were
counted.
Results and discussion
As a result
of the studies, it was found that the development of anthers from different cultivars of Dianthus caryophyllus in vitro
mainly passes through callus forming, i.e. by a way of indirect androgenesis.
At the same time there was not recorded a more desirable way of direct
embryogenesis to form embryoids on anthers.
Anthers
cultivation in nutrient media differing in composition and concentration of
growth regulators showed that the pace and nature of development of new growths
in different carnation cultivars were not the same. Thus, the high frequency of
callus formation (25,7%) was observed in a cultivar Lena, in the medium at a
concentration of phytohormones: 6 BAP – 1 mg/l, NAA – 0,3 mg/l, IAA – 0,5 mg/l
(Table 1). However, the induction of embryonic cell complexes (2,1 %) was
observed on another option of the nutrient medium. The overall incidence of new
growths (47,2 %) in this cultivar was higher than that of the other carnation cultivars.
For the cultivars from Sim group, such as: William Sim, Arthur Sim, White Sim
and Flamingo, an active callus formation was observed in the nutrient medium
containing adenine - 2 mg/l, NAA – 0,5 mg/l, 2,4-D – 3,0 mg/l, IAA – 0,5 mg/l
(15,6 %, 18,9 %, 11,5 % and 13,9 %, respectively). Formation of embryonic cell
complexes in anther culture was more active in the cultivar White Sim (2,7 %)
on the second option of the culture medium. The third group with a high
frequency of callus formation activity included French carnation cultivars:
Cristobal, Carnaval, Amapola, Salome and Vanessa. The best ratio of
phytohormones for them was the option with BAP 6 – 0,5 mg/l, zeatin – 0,1 mg/l.
Table 1. Effect of growth regulators on the initiation of new growths in
anther culture of Dianthus caryophyllus.
|
Genotype |
Induction of
growths, % |
Total, % |
|||||||||
|
Option- 1 |
Option - 2 |
Option -3 |
Option - 4 |
Option -5 |
|||||||
|
Cal-lus |
ECC |
Cal-lus |
ECC |
Cal-lus |
ECC |
Cal-lus |
ECC |
Cal-lus |
ECC |
||
|
Lena |
4,1 |
0,0 |
25,7 |
1,8 |
0,9 |
2,1 |
3,8 |
0,0 |
8,1 |
0,7 |
47,2 |
|
William Sim |
1,9 |
0,1 |
5,9 |
3,1 |
15,6 |
0,4 |
3,2 |
0,0 |
5,3 |
0,0 |
35,5 |
|
Arthur Sim |
4,2 |
0,0 |
7,1 |
0,0 |
18,9 |
0,2 |
2,2 |
0,0 |
3,1 |
0,0 |
35,7 |
|
White Sim |
0,9 |
0,0 |
8,3 |
2,7 |
11,5 |
1,8 |
0,9 |
0,0 |
0,0 |
0,0 |
26,1 |
|
Flamingo |
3,4 |
0,0 |
6,7 |
1,2 |
13,9 |
0,0 |
3,1 |
0,0 |
1,4 |
0,1 |
29,8 |
|
Cristobal |
6,9 |
0,0 |
3,8 |
1,1 |
4,8 |
0,0 |
20,1 |
0,0 |
2,7 |
0,0 |
39,4 |
|
Carnaval |
7,3 |
0,0 |
4,9 |
0,1 |
2,4 |
0,0 |
17,9 |
0,0 |
0,9 |
0,0 |
33,5 |
|
Amapola |
8,2 |
0,0 |
6,5 |
0,0 |
3,9 |
0,0 |
18,5 |
0,0 |
0,0 |
0,0 |
37,1 |
|
Salome |
2,3 |
0,0 |
3,5 |
0,0 |
1,1 |
0,0 |
13,1 |
0,0 |
0,0 |
0,0 |
20,0 |
|
Vanessa |
0,0 |
0,0 |
4,0 |
0,5 |
4,1 |
0,0 |
10,9 |
0,0 |
0,0 |
0,0 |
19,5 |
|
3Ê-84-1 |
2,4 |
0,0 |
4,9 |
0,1 |
1,1 |
0,0 |
5,9 |
0,0 |
3,0 |
0,0 |
17,4 |
New growths with low frequency were
observed in anther culture of the hybrid material. From the eight studied
hybrids, only hybrid 3K-84-1 had callus and embryogenesis.
More than half of the
calluses (58%) had a dense white texture, they quickly turned green in the
light. These calluses were induced from anther culture on the second option of
the culture medium for the cultivar Lena, and on the fourth option for the
cultivars: Cristobal, Carnaval, Amapola, Salome and Vanessa. These calluses had
morphogenic zones. There were also non-morphogenic calluses - loose, watery,
colorless or yellow. The cultivars William Sim, Arthur Sim, White Sim, Flamingo
and almost all hybrids had from 27 to 63 per cent of such calluses. Conducted
cytological control over the processes of embryogenesis in callus culture of Dianthus
caryophyllus isolated anthers showed that callus tissues consist of various
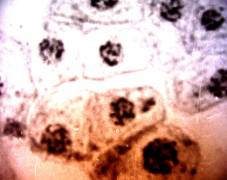
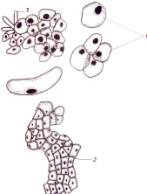
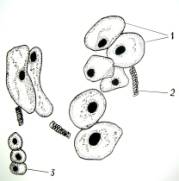
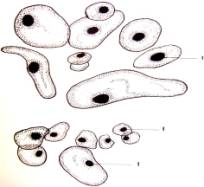
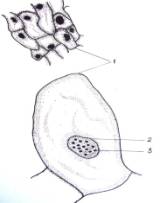
cells in size and shape (Fig. 1. a-i).
|
|
|
|
|
|
|
à |
|
b |
|
c |
|
|
|
|
|
|
|
d |
|
e |
|
f |
|
|
|
|
|
|
|
g |
|
h |
|
i |
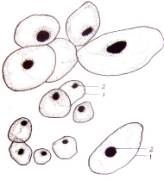
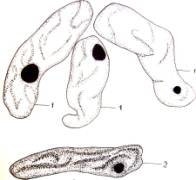
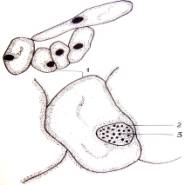
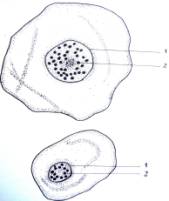
Fig.1.
Callus cells of different shapes and sizes induced from Dianthus
caryophyllus
anthers (by Ryzhkova L.V., Skipina K.P., 1988-1989
yy.): a. - procambial cells of
callus in Carnaval cultivar (increased in 90x10x7); b. -1. callus
cells of Carnaval cultivar, 2.
procambial cells, 3. pollen tubes (increased in 16x10x1,6); c. - 1.
callus cells of Carnaval cultivar 2. - coil vessels, 3. - meristematic cells
(increased in 16x10x1,6); d.e. - callus cells of Cristobal cultivar, 1.,
2 - core (increased in 10x10x1,6); f. - 1. and 2. callus and procambial cell of Cristobal cultivar ( increased
in 40x10x1,6); g. - diploid
callus cell of Carnaval cultivar, 1. - callus cells, 2. - core 3. - chromosomes
( increased in 16õ10õ1,6 è 60õ10õ1,6); h. - diploid and haploid callus
cells of Cristobal, 1. core and 2. chromosomes ( increased in 60x10x1,6); i.
- 1. procambial haploid callus cell of
Carnaval cultivar, 2. - core, 3. - chromosomes.
Except
for large callus cells with loose cytoplasm, in callus tissue derived from
Carnaval and Critobal anthers there were found groups of procambial cells
having substantially rectangular shape and pollen tubes, which were formed
during the germination of pollen that continued to develop in a nutrient medium
until full maturity (Fig. 1 b, d, e). Meristematic hotbeds were also observed
with small, rounded cells with a dense cytoplasm and sieve tubes (Fig. 1c).
Counting
of chromosomes in callus cells of these varieties showed that larger cells had
a diploid number of chromosomes (2n=30), while smaller cells had a haploid set
of chromosomes (n=15) (Fig.1 g, h, i).
With the aim to induce morphogenic
structures in the obtained androgen calluses of D. caryophyllus they
were passaged to special, so-called regenerative medias with higher cytokinin
content, compared to auxin,: 6-BAP- 1,0 mg/l, zeatin- 0,5 mg/l, NAA - 0,1 mg/l.
After 21 days of cultivation of
callus, induced from carnation anther of Lena cultivar, we could observe the
morphogenic structures from which we subsequently obtained regenerated plants.
Conducted cytological analysis of the regenerated plants showed that they had a
diploid set of chromosomes (2n = 30).
Conclusions
It is revealed that genotype has the
greatest impact on the efficiency of anthers culture in vitro. According
to the research of different Dianthus caryophyllus genotypes on
responsiveness to in vitro cultivation conditions, cultivars with high
androgenic potential were recorded. They include the following cultivars:
Cristobal, Carnaval, Amapola, Salome and Lena. It has been established that the
ability to induce proliferation processes, the intensity of their transmission
and then the regeneration also depend
on the composition and concentration of phytoregulators.
References
1.
Dogramaci-Altuntepe M., Peterson T.S.,
Jauhar P.P. Anther culture-derived regenerants of durum wheat and their
Cytological characterization // The American Genetic Association. – 2001. – 92.
– P. 56–64.
2.
Hassawi D.S., Liang G.H. Effect of
cultivar, microspore development of anther culture of wheat and Triticale //
Plant Breeding. – 1990. – 105. – P. 332–336.
3.
Murigneux A., Bentolila S., Hardy T. et
al.Genotype variation of quantitative trait loci controlling in vitro
androgenesis in maize // Genome. – 1994. – 37. – P. 970–976.
4.
Babbar S.B., Agarwa A.K. Sahay Sh.
Isolated microspore culture of Brassica:An experimental tool for development
studies and crop improvement // Indian Journal of Biotechnology. – 2004. -3,
April. – P. 185–202.
5.
Kurt O., Evanth G.M. Anther Culture
Potential of Linseed (Linum usitissimum L.): Effects of Genotypes and
Pretreatment on Callus Formation and Differentiation // J.of Agriculture and
Forestry. – 1998. – 22. – P. 553–560.
6.
Clapham D. In vitro developmant of callus
from the pollen of Lolium and Hordeum // Z.Pflanzenzuchtg. – 1971. – 69. – P.
142–155.
7.
Kalashnikova Ye.A., Chung May Dyk
Theoretical and practical aspects of obtaining haploid and dihaploid plants of
different Brassica species in vitro. - LAP: LAMBERT Academic Publishing, 2012,
126 p.
8.
Savelyev N.I., Oleinikova O.Ya, Zhukov
O.S. Androgenesis in apple culture in vitro. Agricultural Biology, 1999, ¹ 3,
p. 48-53.
9.
Mireille Sitbon Production of haploid
Gerbera jamesonii plants by in vitro culture of unfertilized ovules. Agronomie,
1981,1(9), 807-812.
10. Luckett D.J. Doubled haploid production by antherculture for Australian
barley breeding // Austral. J. Agr.Res. – 1992. – 1. – P. 67–78.
11. Castillo A.M., Vallés M.P, Cistué L. Improvement ofbarley
androgenesis in breeding // Biotechnologicalapproaches for utilization of
gametic cells. –Luxemburg : Office for Official Publications of theEuropean
Communities, 2001. – P. 15–21.
12. Barnabas B. Protocol for producing doubled haploid plants from anther
culture of wheat (Triticum aestivumL.) // Doubled haploid production in crop
plants. –Dordrecht : Kluw. acad. publ., 2003. – P. 65–70.
13. Øåñòîïàë Î.Ë., ²ãíàòîâà Ñ.Î. Öèòîëîã³÷íèé ìîí³òîðèíã åôåêòèâíîñò³
ìîðôîãåíåçó â êóëüòóð ïèëÿê³â ì’ÿêî¿ ïøåíèö³ äëÿ îö³íêè ¿¿ çäàòíîñò³
äîàíäðîãåíåçó // Ôàêòîðè åêñïåðèìåíòàëüíî¿ åâîëþö³¿ îðãàí³çì³â. – Ê.: Ëîãîñ,
2006. – Ò. 3. – C. 527–531.
14. ForoughiWehr B., Friedt W., Wenzel G. On the geneticimprovement of
androgenetic haploid formation inHordeum vulgare L. // Theor. Appl. Genet. –
1982. –62. – P. 233–239.
15. Barnabas B. Anther cultture of maize (Zea mays L.) Doubled haploid
production in crop plants. – Dordrecht : Kluw. acad. publ., 2003. – P. 103–108.
16. Butenko R.G. Cell biology of higher plants in vitro and biology and on
their basis: Textbook. - M: FBC - PRESS, 1999., With -160.
17. Pausheva Z.P. Workshop on Plant Cytology / Z.P. Pausheva. - M.:
Agropromizdat, 1988. – 271 p.