Shakin E. S., Nikityuk V. G., Ph.D., Yarnykh Ò. G., Professor
National University of
Pharmacy, Ukraine (sh.e.s@list.ru)
Structuring
of Pharmaceutical Development Processes within the Pharmaceutical Quality
Systems
Modern system of the Pharmaceutical Quality Assurance is based on PQS –
Pharmaceutical Quality System, which was formulated in 2008 by ICH (International Conference on Harmonization of
Technical Requirements for Registration of Pharmaceuticals for Humane Use),
and was introduced as compulsory constituent for the rules of Good
Manufacturing Practice since 2013, having included all stages of drugs
lifecycle beginning with Pharmaceutical Development into the sphere of GMP. For
this, any pharmaceutical company should extend Quality System application in on
its Research and Development (R&D) department (laboratories), which should
follow procedures in their work.
One of the fundamentals principles of the Pharmaceutical Quality System
is: “product
and knowledge management should be done at all stages of the product life cycle”.
Application of Knowledge Management system (process) at the stage of
pharmaceutical development should be focused on obtaining the fullest scope of
knowledge about the product and building in quality (in the full sense of the
drug quality, including safety) into the product being created resting upon
this knowledge.
ICH Q10 Guideline define following elements of pharmaceutical
development process as a stage of product lifecycle:
- development of
medicinal product (including active ingredient);
- development of
medicinal product’s formula and “container / closure” system;
- production of
experimental medicinal product;
- development of
delivery system (if necessary);
- development of
production process (process technology) and its scaling;
- development of
analytical procedures.
Each component within PQS should be implemented in compliance with
written procedures according to the internal system requirements of the good
documentation; provisions of the ICH Q8 Guideline concerning the pharmaceutical
development itself should be taken into account too.
The mentioned above components are applicable to all medicinal products,
including those for solid dosage forms. Particularity of solid dosage forms’
production – tablets, hard gelatin capsules, effervescent tablets, coated
tablets, etc. – requires special attention during the pharmaceutical
development (as well as during all subsequent stages of the products lifecycle
in these dosage forms) due to the complexity of the production process, its
multi-stage and significant variability.
Taking into account the above-mentioned, the authors suppose that the
universal approach for defining components of pharmaceutical development stage
requires more precise structuring. Such structuring was formed by the authors
during identification of practical approaches for documenting the
pharmaceutical development process as the Pharmaceutical Quality System
Process. Part of this structure is schematically shown below in figure 1 as
main components of the pharmaceutical development process.
The concept of the author's approach to the interpretation of the
requirements of the ICH Q10 Guideline, provisions of the ICH Q8 Guideline and
GMP rules is based on emphasizing of 3 key subprocesses: development of
medicinal products, development of product receipt technology and development
of analytical procedures. Detailed components of each of these subprocesses
have been expanded by such components as:
- development of
production formulation / formula that is not only the definition of structure
and information in the dossier for marketing authorisation (MA dossier) but
also the basis for development of industrial production technology and
regulation of series size;
- definition /
development of equipment and production lines cleaning methods after the
product production as a key factor in ensuring safety of medicinal products on multiproduct lines and also
development of analytical procedures of cleaning quality control;
- analytical methods’
validation, without which it is impossible to
form
the MA dossier correctly, but also to move to the next stage of the product
lifecycle – transfer.
The offered structuring of the pharmaceutical developments procedures
provides the rational algorithm of creating the quality system documentation
for the whole process, simplifies the possibility of detailed regulation of
each procedure in the appropriate writing techniques, their relationship with
each other and, ultimately, properly documenting of all procedures for building
in quality into the developed medicinal product.
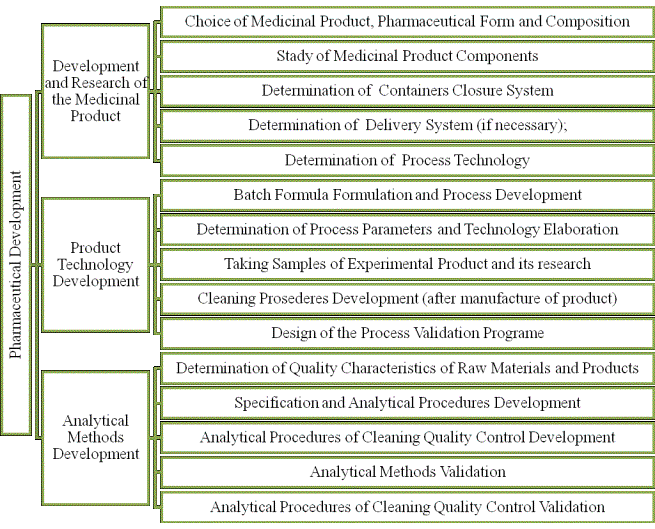
Figure 1. Main components of the
pharmaceutical development process