Kisteniuk
N.S., Skrotska O.I.
National University of Food
Technologies, Ukrainian
Tyloronu
analogs as antiviral agents
Years
of experience in the clinical use of interferon (IFN) preparations revealed
their effectiveness for prevention and treatment. However, in clinical use of
these drugs was found deficiencies of their application.
Ø
Formation of anti-interferon
antibodies that neutralize exogenous IFN.
Ø
With an overdose of exogenous IFN
may have side effects.
Ø
Rates of treatment by preparations
IFN are extremely expensive [1, 2].
The main method to
eliminate such shortcomings is the use of interferon inducers (IIFN). The most known
representative of inducers IFN is synthetic tilorone.
(2,7-bis[2-(diethylamino)ethoxy]-9H-fluoren-9-one)
Figure 1. In Ukraine first tiloron was synthesized O. Bogatsky and staff by
technology [3]. And implemented by into medical practice as the drug Amixin IS (JSC
"InterChem").
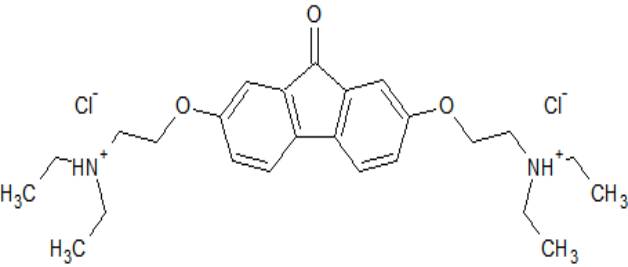
Figure 1.
2,7-bis[2-(diethylamino)ethoxy]-9H-fluoren-9-one
Predecessor
of tilorone is fluorene Figure 2 and fluorenon Figure 3, which is not able to
of inducing the synthesis of IFN. However, derivatives of fluorene and
fluorenon turned biologically active substances that suppress the development
of many virus infections [1].
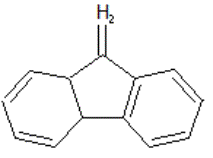
Figure 2
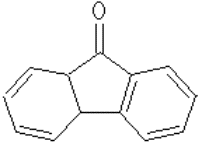
Figure 3
Were
carried out numerical studies of new derivatives tilorone. As a result, the
study of derivatives 28 tilorone antiviral activity detected in dihydrochloride
bis [dybutylaminopropil] -9 oksofluoren--2,7-dicarboxylate Figure 4.
Y = СОО
X = -(СН2)2
R' = n-С4Н9
Q = О
|
|
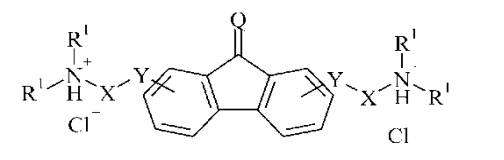
Figure 4. dihydrochloride bis [dybutylaminopropil] -9
oksofluoren--2,7-dicarboxylate
Also
is described is shown interferonindukuyucha synthesis and active in of
2,7-bis-2 (4-aza-tricyclo [4,3,1,13'8] -undets-4-yl)-9H-ethoxy fluo- ren-9-one
Figure 5. Same time is not fixed interferonindukuyucha activity in vitro
separate from the nucleus or side chain fluorenonovoyi system. Recently shown
erferonogenie activity glycoside derivative fluorenolu-9, and the authors
associate it with intercalation ability of the drug [4].
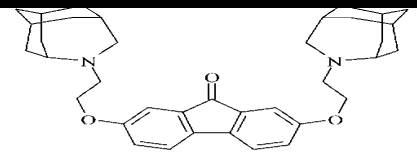
Figure 4. 2,7-bis-2(4-aza-tricyclo [4,3,1,13'8] -undets-4-yl)-9H-ethoxy
fluoren-9-one
So, for the manifestation
interferonindukuyuchoyi and antiviral activity of these compounds necessary
condition is the presence of side chain molecules of the two groups with basic
properties (eg. amines) and the presence of a central system lipophilic
aromatic nature. But, later found compound 2,7-dietoksyfluorenonu who had high
interferonindukuyuchu activity Figure 6 and compound - 2,7-di (adaman- tan-1
oyiloksy) fluorenonu Figure 7, which had high antiviral activity. Therefore the
precondition is reduced to nothing regarding of basicity of side chains [1].
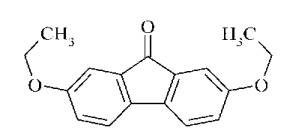
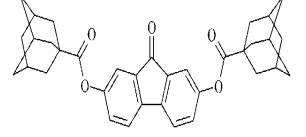
Figure 6.
2,7-dietoksyfluorenonu
Figure 7. 2,7-di (adaman-
tan-1 oyiloksy) fluorenonu
In particular, the conditions shown in
vitro antiviral and immune-modulating activity for 9 fluorenon-4-carboxamides
Figure 8 [6], β-O-glucosides 9 fluorenon-2-karbohidrokyeesteriv [7],
2/4-substituted-9 -fluorenoniv and their O-glucosides [8].
глюкозидів [8].
|
Тable 1. 9 fluorenon-4-carboxamides
|
|
Сотр.
|
К
|
Сотр.
|
К
|
|
1
|
(СН2)2ОН
|
6
|
(СН2)2N(СНз)2
|
|
2
|
(СН2)3ОН
|
7
|
(СН2)зN(СНз)2
|
|
3
|
СН2СНОНСН3
|
8
|
(СН2)2N(СН2СН3)2
|
|
4
|
(СН2)4ОН
|
9
|
 (СН2)2N O (СН2)2N O
|
|
5
|
(СН2)2О(СН2)2ОН
|
10
|
 (СН2)3N O (СН2)3N O
|
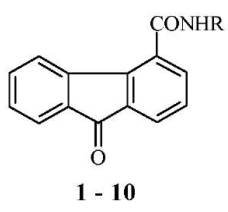
Figure 8. 9 fluorenon-4-carboxamides
In
a study conducted in the Department interferon problems and immunomodulators
Institute of Microbiology and Virology. DK Zabolotnogo NAS of Ukraine, was
conducted a large-scale screening of compounds which resulted in been selected
most active and least toxic compounds Figure 9, 10.
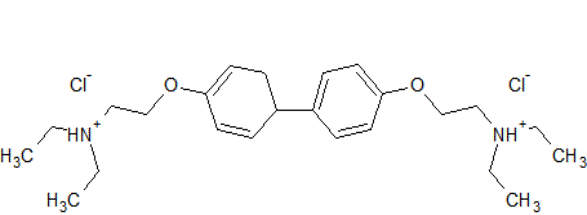
Figure 9. 4,4-bis
[2 (diatylamino) ethoxy] biphenyl dihydrochloride
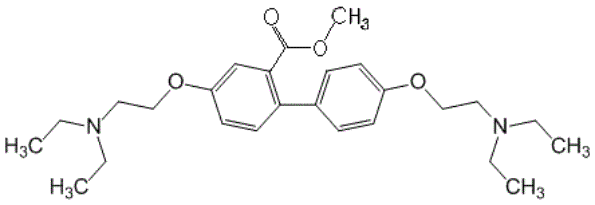
Figure 10. 2-methoxycarbonyl-4-4
bis [2 (diatylamino) ethoxy] biphenyl dihydrochloride
Biphenyl derivatives relate up to
amphiphilic compounds with clearly established polar and nonpolar domains. Their
molecules include nonpolar hydrophobic domain, which includes aromatic rings,
and two are symmetrical hydrophilic lateral chains are positively charged under
physiological pH. This chemical structure allows the investigated compounds
effective impact on cell surface. Currently IIFN have taken a worthy place in
the therapy of virus infections as antiviral drugs and correctors immunity [9].
Were synthesized numerous series of
heterocyclic analogues tilorone bis-base (Figure 11) carbazole, dibenzofurans,
dybenzotiofeny; phenanthrene, fenantrydyny, dybenzopirany, xanten and
acenaphthene [10].
|
Тable
2 bis-base analogues tilorone
|
|
Сотр.
|
Назва
|
К
|
|
1
|
(1) karbozoly
|
X
= NН
|
|
2
|
(1)
dibenzofurans
|
X
= O
|
|
3
|
(1) dibenzotiofeny
|
X
= S
|
|
4
|
(2) fenanthrene
|
X
= CH
|
|
5
|
(2)
fenantrydyny
|
X
= N
|
|
6
|
(3)
dybenzopirany
|
X
= O
|
|
7
|
(4) ksksanteny
|
X
= O
|
|
8
|
(1)
dimethylamino acetyldybenzotiofen
|
X
= S
R = -СО-СН2-N(СН3)2
|
|
9
|
(1)dimethylamino
acetyldybenzofurans
|
X
= О
R = -СО-СН2-N(СН3)2
|
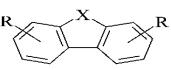
1
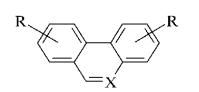
2
3
4
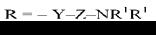
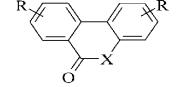
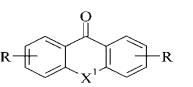
де Y = О; СН2;
СО; СОО; S;
СS;
СS0;
Z
= (СН2)n;
R1
- alkyl, або NR1R1- N – containing
heterocycle
Figure 11. bis-base analogues tilorone
Among them the most interferonindukuyuchu
activity showed diethylaminopropilfluoranten (Figure 12), and dimethyl dimethylaminoatsetyldybenzot
aminoatsetyldybenzofuran [11].
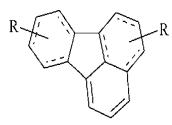
Figure 12.
diethylaminopropilfluoranten
Thus,
today a large number of have been synthesized derivatives tilorone which can be
used as of inductors IFN, but only some of them were appropriate for clinical
use. Most of inductors IFN
through toxicity, lack scrutiny of and high cost in medical practice is not
used. Therefore an urgent task today is the search and creation drugs
interferon of inductors deprived of their mentioned deficiencies.
References:
1.
Співак М.Я., Жолобак
Н.М., Ляхов С.А., Карпов О.В. Індуктори інтерферону як противірусні антигени:
нові аспекти старої проблеми // Журн. орган. та фармац. хімії. – 2007. – Т.5, №
17. – С. 4- 20
2.
Pestka S, Crommelin J. Interferons and interferon
inducers. // Pharm. Biotechnol. –2013. –
Vol. 6. – Р. 413-437
3.
Богатський
О. В. Про синтез 2,7-біс-[2-(диетиламіно)етокси]- флуорен-9-ону
// Доповіді АН УРСР, сер. Б. – 1976. – №7. – С. 610-612.
4.
Alcaro S., Arena A.,
Neri S., Ottana R., Ortuso F.,Pavone B., Vigorita M.G. Design and
synthesis of DNA-intercalating 9-fluoren-beta-O-glycosides as potential
IFN-inducers, and antiviral and cytostatic agents // Bioorg. Med. Chem. – 2004.
– Vol. 12, No. 7. – P. 1781-1791.
5.
Tkachenko I., Garkava
K., Karpov O. Molecular complex, yeasts RNA – tilorone hydrochloride,
interferon i indueer – immunotropic properties // VISNYK OF L’VIV UNIV. –2005. – Vol. 39. – Р. 141-147
6.
Alcaro
S., Arena А. 9-Fluorenon-4-carboxamides:
synthesis, conformational analysis, anti-HSV-2, and immunomodulatory
evaluation. Note II // ARKIVOC. – 2004. – Vol. 5. – P.
334-348.
7.
Alcaro S.H., Arena G.А. Biocatalysed synthesis of (3-O-glucosides from 9-fluorenon-2-
carbohydroxyesters. Part 3: IFN-inducing and anti-HSV-2 properties // Bioor.
Med. Chem. – 2005. – Vol. 10. – P. 3371-3378
8.
Arena
А., Ciurleo D.R. 2/4-substituted-9-fluorenones and their O-glucosides as potential
immunomodulators and anti-herpes simplex virus-2 agents. Part 5 // Eur.
J. Med. Chem.
– 2008. – Vol. 12. –P.
2656-2664.
9.
Співак М.Я., Жолобак
Н.М., Богород-Кобельська О.С., Оленівська З.М. Активність похідних
дифенілу на різних модельних системах вірус-клітина // Фізіол. журн. – 202. –
Т.58, № 1. – С. 36-42
10. Карпенко А.С., Шибинская М.О., Ляхов С.А.,
Жолобак Н.М., Олевинская З.М., итвинова Л.А., Спивак Н.Я., Андронати С.А. Синтез,
ДНК-связывающие и интерферониндуцирующие свойства гидразонов изатина и
бензоизатина // Хим. фарм. журн. –2006. – Т. 40, № 11. – C. 15-22.
11. Ляхова Е.А., Ляхов С.А., Литвинова Л.А.,
Андронати С.А., Лебедюк М.Н., Федчук В.П., Хорохорина Г.А. Синтез и
ДНК-связывающие свойства аминоацетилгидразонов 9-формилакридина и 9-форми
лан-трацена // Хим.фарм. журн. – 2005. – Т. 39, № 4. –С. 16-20














