Технические науки/1. Металлургия
A.R. Toleuova1, D.U. Smagulov1,
A.A. Amenova1
1- Kazakh National Technical University after K.I. Satbayev, 050013
Almaty, Kazakhstan
On the calculation and representation of multicomponent systems
Introduction
Casting
alloys are called, are used for the production of shaped castings. Casting
aluminum alloys - widespread (mainly construction) materials consumption is
growing every year in almost all sectors of modern industry.
Feature
of the production of cast aluminum alloys is the high proportion of recycled
materials used. Since only 70 - 85% by weight of the charge for melting of cast
aluminum alloys are waste and scrap, which is several times larger than the
corresponding figure for deformable aluminum alloys.
Basic
requirements for cast aluminum alloys - is a high level of performance (mechanical
and corrosion properties) combined with good processability during mlding. Last
for the currently used technologies in the industry means lower propensity to
hot (crystallization) cracks, good fluidity, minimal shrinkage porosity, ie
good casting properties.
In
the various branches of technology is now used dozens of cast aluminum alloys. Patented formulations of thousands of alloys.
However, almost all of them contain a relatively small number of alloying
elements. All the alloying elements that make up the cast aluminum alloys can
be divided into three groups: the main alloying elements, small additions and
impurities. The same elements can belong to different groups depending on the
alloy.
The
first and main function of the alloying elements - to increase the strength of
aluminum (pure aluminum is too low strength - sв < to 60 MPa). Hardening is
achieved by formation of solid solution, and - in many systems - through
precipitation hardening. In addition, the content of alloying elements depends
on the properties of casting alloys, which largely determines their technology
and, consequently, the degree of industrial use.
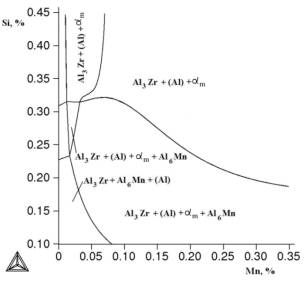
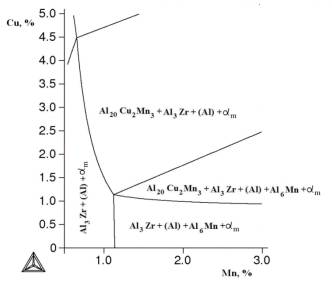
Figure 1. Isothermal section of Al-Cu-Mn-Zr -
Fe - Si at 0,3% Fe, 0,4% Zr at 540 °C.
The
experimental part
Obviously,
to make informed choices concentrations of impurities, alloying elements and
heat treatment, a careful analysis of Al-2Cu-1, 5Mn-0, 4Zr - 0,1 ÷ 0,3
Fe - 0,1 ÷ 0,3 Si. Therefore, in this paper has been tasked to perform
such an analysis using modern software Thermo - Calc. This program allows you
not only to build almost any size, but to count on a quantitative level the
phase composition of the alloy at different temperatures (including the mass
and volume fractions of phases and the concentration of these elements). No
calculation to get this information is practically impossible.
The
addition of zirconium binary alloys have been known to lead to the formation
phase Al3Zr. Although the literature there are no data on the
structure of the diagram Al-Cu-Mn-Zr-Fe-Si, distribution of phase domains in
the aluminum corner of the system in the solid state can be predicted based on
available information. It is known that zirconium greatly increases the
liquidus temperature in binary alloys. It is also known that adding zirconium
improves the resistance of various types of corrosion. At the same time be sure
to take into account that the positive effect of zirconium can be achieved only
in strict compliance with technology. Otherwise, its presence in the alloy may
be useless and even harmful. For example, if the temperature of the melt with
this addition, the input, usually from the ligature, was too low, then the
structure might have a rough castings of primary aluminides, which reduce the
mechanical properties.
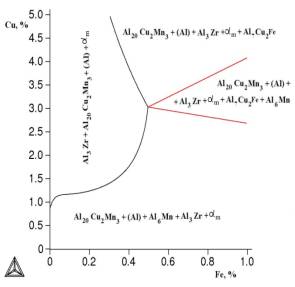
Figure
2. Isothermal section of Al-Cu-Mn-Zr - Fe - Si at 1,5% Mn, 0,3% Si at 540 °C.
In
the non-equilibrium solidification conditions the solubility of manganese in
aluminum increases, and the formation of a ternary compound is suppressed.
Therefore, in these alloys, together with (Al) phase coexist Al2Cu
and Al6Mn. After the formation of primary crystals (Al), there is an
evolution of phases and Al2Cu Al20Cu2Mn3
by the following reaction: L ® (Al) + Al2Cu + Al20Cu2Mn3
at 547 ° C. With further increase of the concentration of copper significant
changes were observed.
Aluminum
angle of the phase diagram characterized by a rather complex structure. In
equilibrium with the aluminum solid solution phases, except for the binary
systems (Al6Mn and (Si)) is a ternary compound αm
(Al15Mn3Si2). In the non-equilibrium
solidification conditions in aluminum alloys can also be present phase Al4Mn
and Al10Mn3Si.
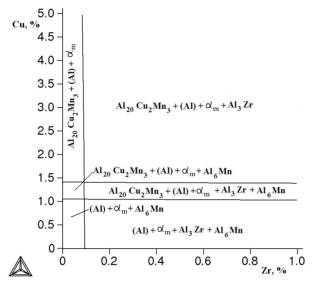
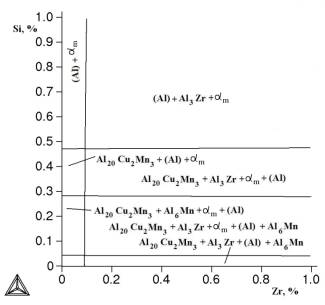
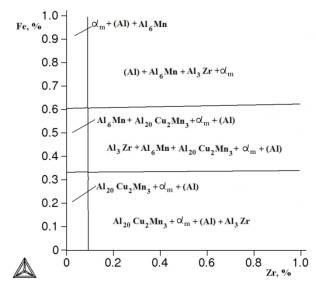
Figure
3. Isothermal section of Al-Cu-Mn-Zr - Fe - Si at 0,3% Fe, 1,5% Mn at 540 °C.
Below
is a nonvariant reaction that may occur in the system Al-Cu-Mn-Zr - Fe - Si: L ®
b(Al) + Si + αm at 0,3% Fe, 0,2% Mn and ~
575 ° C.
Phase
Al15Mn3Si2 (26,3% Mn, 8,9% Si), denoted as and
Al10Mn2Si, Al12Mn3Si, Al9Mn2Si1,
8, α (MnSi) or αm, there is a homogeneity range 25 - 29% Mn, 8 - 13 %
Si. Phase αm is skeletal form and has a less harmful effect on the
mechanical properties of the alloy as compared to other phases. Primary
crystals of this phase have the form of equiaxed polyhedra, they often form
clusters. This phase has a cubic lattice (space group Pm3, 138 atoms per unit
cell) with a = 1.265 - 1.260 nm . The density of 3.55 g/cm3 phase,
while the microhardness at room temperature, 8.8 GPa. Silicon is slightly
soluble in phase Al6Mn. The solubility of manganese in the phase Al15Mn3Si2
is 0.7 - 0.8%.
Table
1.
The
parameters characteristic of the crystallization of alloys of the Al-Cu-Mn-Zr -
Fe – Si
|
Fe, % |
Si, % |
Cu, % |
tL, °C |
tS, °C |
Phases |
|
0,1 |
0,1 |
0,5 |
790 |
639 |
(Al) +Al6Mn +Al3Zr |
|
0,3 |
0,3 |
5 |
788 |
557 |
(Al) +Al20 +Al3Zr + αm |
Liquidus
temperature (TL) and solidus (TS) is one of the most
important characteristics of any alloy. With these temperatures determine the
modes of heat treatment temperature of melting and casting alloys. The results
of calculating the values of TL and TS
for the alloy of Al - Cu - Mn - Zr-Fe - Si are shown in Table. 1. Based on the
calculation results we can conclude that copper does not affect the TL,
but significantly reduces the TS. On the other hand, iron and
silicon in small quantities do not significantly affect the liquidus and
solidus.
Conclusions.
Using the program Thermo-Calc quantitative analysis of the phase diagram of Al
- Cu - Mn - Zr - Fe - Si (isothermal sections, temperature, solidus and
liquidus).
The
composition of this alloy has a very narrow limits. Copper is added to reduce
the pitting corrosion. Allowed up to 0.5% iron and silicon, which leads to some
strengthening of the alloy, without significant loss of corrosion resistance.
Under equilibrium conditions present in the alloy phase Al15Mn3Si2,
which does not exert a strong influence on the mechanical properties of the
alloy.
References
1.
Mondolfo LF Structure and properties of aluminum alloys .- Moscow: Metallurgiya
(1979).
2.
Industrial Aluminum Alloys: A Handbook / Ed. FI Kvasov, JH Friedländer.
Moscow: Metallurgiya, 1984.
3.
Aluminium alloys for bearings and their application. Sat papers edited by MM
Khrushchov. - Moscow: USSR Academy of Sciences, 1954.
4.
22. Bullock, AE Structure and Properties of Binary Metallic Systems. Volume 1 -
M. Fizmatizdat, 1959.
5.
Properties of Aluminium Allois: Tensile, Creep and Fatigue Data at High and Low
Temperatures / Ed. by J. Gilbert Kaufman. - ASM International and The Aluminium
Association 1999.
6.
Aluminum. Properties and physical metallurgy: Ref. ed. / Ed. JE Hatch: Trans.
from English. - Moscow: Metallurgiya, 1989. - 324s.
7.
The use of aluminum alloys: Ref. ed. / Altman MB, Arbuzov, Yu.P. and others -
Moscow: Metallurgiya, 1985. - 344.
8.
Stroganov, GB High-strength cast aluminum alloys. - Moscow: Metallurgiya, 1985.
- 216s.
9.
GB Stroganov, VA Rotenberg, Gershman GB Aluminum alloys with silicon. - Moscow:
Metallurgiya, 1977. - 271s.
10.
Zakharov, AM Industrial non-ferrous alloys. Phase composition and structural
components. - Moscow: Metallurgiya, 1980. - 256 p..