Localization of Lipoxygenase in the Cells of the Young Ovule of Larix kaempferi (Lamb.) Carr.
Aleksandra
Seta1, Ewa Szczuka1, Jarosław Pawelec2,
Ewa Skórzyńska-Polit3, Irena Giełwanowska4,
Marcin Domaciuk1
1Department
of Plant Anatomy and Cytology, Maria Curie-Skłodowska University,
Akademicka 19, 20-033 Lublin, Poland
2Department
of Comparative Anatomy and Antroplology, Maria Curie-Skłodowska
University, Akademicka 19, 20-033 Lublin, Poland
3Department
of Molecular Biology, John Paul II Catholic Univesity in Lublin, Kraśnicka
Av.102, 20-718 Lublin, Poland
4Department
of Plant Physiology and Biotechnology, University of Warmia and Mazury,
Oczapowskiego 1A, 10-719 Olsztyn, Poland
ABSTRACT
The localization of the enzyme lipoxygenase (LOX; EC 1.13.11.12) was investigated in the cells of the ovule of Japanese larch Larix kaempferi (Lamb.) Carr. at the stage of megaspore mother cell in the nucellus. In the presented investigations the following methods were used: light microscopy, electron microscopy, and the immunogold method. The immunogold labelling technique was used for precise localization of LOX on the cytological level. The following parts of the young ovule were examined with an electron microscope: the integument, the nucellus, and the megaspore mother cell.
The immunogold labelling method demonstrated that LOX occurred most often in two cell elements: the cytosol and vacuoles. The immunogold particles were usually distributed randomly in both parts of the cells of the three investigated ovule parts: the integument, the nucellus, and the megaspore mother cell. In smaller quantities, the immunogold particles which revealed the presence of the enzyme were found to be associated with membranes: the plasma membrane, short endoplasmic reticulum cisternae – mainly RER (rough endoplasmic reticulum), and the nuclear envelope. LOX was also detected near mitochondria and plastids. Additionally, some single immunogold particles were observed at or in the area of the cell walls and nuclei of the ovule cells.
The immunolocalization of LOX in an
electron microscope indicated a similarity in the distribution of lipoxygenase
in all cells of the particular investigated larch ovule parts to its
distribution in other, earlier investigated plant cells. However, the
immunogold reaction in the cells of the larch ovule was less intense in
comparison with other plant organs.
INTRODUCTION
The species whose ovules are the object of the
investigations presented in this paper is the gymnosperm tree Larix kaempferi (Lamb.) Carr.. The tree belongs to the family Pinaceae. This species of larch is also called Larix
leptolepis or Japanese larch because of its Japanese origin. Though native to Japan, L. kaempferi
is also widely planted in other parts of the world as a forestry tree due to
its strength and vigour. It is hardy in zones 5–6. L. kaempferi is similar to European larch in
size and has bluish–green needle–like leaves
that, like in a handful of other deciduous conifers, turn yellow in autumn. Its
tiny young cones are purplish or pinkish, as in most Larix species. The wood of L. kaempferi is tough and durable, and
is mostly used for general construction work. Small
larch poles
are widely used for rustic fencing.
The
enzyme which was localized by us in the young ovule of L.
kaempferi was lipoxygenase. It is well known that immunolocalization of lipoxygenase in an electron microscope
indicates a functioning „lipoxygenase pathway” in all cells of the investigated
angiosperm plant parts. The intensity of the immunogold reaction may indirectly
indicate differentiated activity of the enzyme in particular plant cells (Bowsher et al., 1992; Feussner and
Wasternack, 2002, Feussner et al., 1995;
Schmitt and van Mechelen, 1997; Skórzyńska-Polit
et al., 2005; Skórzyńska-Polit
et al., 2006; Szczuka et al., 2004; Szczuka et al., 2006; Szczuka et
al., 2007; Szczuka et al.,
2008; Szczuka
et al. 2008, in press).
Lipoxygenases (LOX; EC 1.13.11.12) are nonheme iron-containing dioxygenases
widely distributed in plants, animals and fungi. Basically, LOX catalyzes the
addition of molecular oxygen to polyunsaturated fatty acids to produce an
unsaturated fatty acid hydroperoxide. The hydroperoxy fatty acid products of
the LOX reaction can be further
converted to different compounds through the action of enzymes participating in
at least six pathways (for the proposed scheme of possible
“lipoxygenase pathway” and references see an interesting paper by two Mexican
authors Porta and Rocha-Sosa, 2002, and Szczuka et al., 2007).
In plants, products of the LOX pathway have several
diverse functions, which have been described, among others, by the following
authors: Feussner et al. (1997),
Lima de Carvalho et al. (1999),
Lynch and Thompson (1984), Rosahal (1996), and Porta and Rocha-Sosa (2002). The functions are numerous and are fulfilled
in the seed, during germination, vegetativ growth, or after sustaining a wound
from a herbivore attack or pathogen attack (for a table with a detailed list of
active roles of LOX in several processes during plant life see Porta and Rocha-Sosa (2002), and Szczuka
et al. (2007)).
Lipoxygenase is investigated mainly with
physiological and genetic methods. We undertook the study of lipoxygenase
because of a gap in the knowledge concerning the localization of lipoxygenase
on the cytological level. The investigations we present in this paper concern
the localization of the enzyme lipoxygenase in the cells of the developing
ovule of the Gymnosperm tree, larch L. kaempferi and represent a continuation of our several
year research of the lipoxygenase enzyme in different plant organs.
MATERIAL AND METHODS
Plant material
Seed scales from female cones
of Japanese larch Larix kaempferi (Lamb.)
Carr. growing in the Botanical Garden
of Maria Curie-Skłodowska University in Lublin (eastern part of Poland)
were used in the study. Starting from March 2006, single ovules of L. kaempferi had been isolated from seed
scales.
Light and electron microscopy
Single ovules, freshly excised from the seed scales, were fixed in 3.5% glutaraldehyde in 0.05 M cacodylate buffer, pH 7.0 for 24 h at room temperature. The samples were postfixed in osmium tetroxide (OsO4), dehydrated in ethanol and aceton, and embedded in Spurr’s resin. The material was cut into semi–thin sections (1–2μm thick) and put on slides. Then, the semithin sections of plant organs were stained with 0.1% toluidine blue in 0.5% sodium carbonate at about 60°C and observed in the light microscope in order to recognize the stage of development of the young ovule of L. kaempferi.
Immunolabelling
For
immunogold labelling, the young larch ovules were cut from the seed scale and fixed in 2% formaldehyde (necessarily
freshly prepared from paraformaldehyde) and 1% glutaraldehyde dissolved in PBS
(0.1 M phosphate buffer, pH 7.4) for 24 h at 4oC. The samples were
rinsed several times (four to five) in PBS and 0.5 M NH4Cl in PBS,
dehydrated in ethanol, embedded in LR White resin (Sigma), and polymerised at
60oC overnight. Ultrathin sections were collected on nickel grids,
treated with aqueous 0.56 M sodium periodate for 30 min, thoroughly washed with
distilled water, and treated with 0.1 M HCl for 10 min followed by a 5 min
water wash. The sections were incubated first in 1 % BSA in PBS for 30 min at
room temperature, then with preimmune rabbit serum (Agrisera) diluted 1/1000 in
PBS–BSA for 1 h at room temperature. After three times washing with PBS–BSA
(each wash lasting 10 min), the sections were incubated with PBS-BSA containing
rabbit anti–LOX antiserum diluted 1/1000 for 1h and repeatedly washed with
PBS–BSA. Goat anti-rabbit immunoglobulins conjugated to 10 nm gold particles
(GAR–gold) (Sigma) were diluted 1/50 in PBS–BSA and then applied for 40 min at
room temperature. Next, the sections were washed several times with PBS and
redistilled water. As an additional control, samples were incubated with
preserum and GAR–gold or GAR–gold alone, omitting the primary antiserum. The
sections were stained with 2 % uranyl acetate for 5 min and with Reynolds
reagent (lead nitrate and sodium citrate) for 1 min. All the sections were
examined using the transmission electron microscope.
RESULTS
Larix kaempferi (Lamb.) Carr. (Fig. 1) is a
medium-sized (about 10–15 m tall) decidous coniferous tree. Its crown is broad
conic; both the main branches and the side branches are level. The shoots are
dimorphic, with growth divided into long shoots (typically 10-50 cm long) and
bearing several buds, and short shoots (only 1–2 mm long) with only a single
bud. The leaves are needle–like, light glaucous green, 2–5 cm long; they turn
bright yellow to orange before they fall leaving the pinkish–brown shoots bare
until the next spring. The cones (Fig. 4) are erect, ovoid-conic, 2–3.5 cm
long, with 30–50 reflexed seed scales. The immature cones are green and turn
brown at maturation. After 4–6 months from pollination, the seeds are released
from the cones. In the investigated species of larch, the old cones (Fig. 2) commonly remain on the
tree for many years. The trunk is covered with bark as shown in Figure 3.
In
Polish climatic conditions, female cones of L.
kaempferi appear in March. The morphological structure of the female cone
is shown in Figure 5. The female cone is built from bigger bract scales and
much smaller, at this stage of development, seed scales (Fig. 6). Figure 6
shows the stage of seed scale development at which the localization of
lipoxygenase was examined with the electron transmission microscope (TEM) in
this paper.
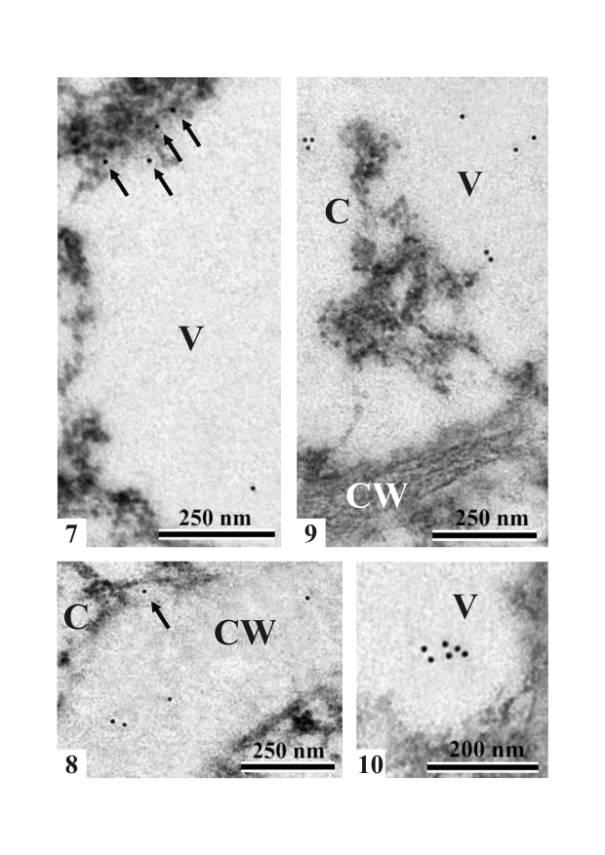
Immunogold
LOX PAb localization in the cells of the ovule nucellus of L.
kaempferi (at the stage of
megasporocyte in the nucellus) showed single gold particles in the cytoplasm,
in the vacuole (Fig. 7), and in the area of the cell wall (Fig. 8). The single
gold particles were found mainly at the edge of the cytoplasm and vacuole.
Rarely, single particles were visible at the edge of the cell wall and
cytoplasm. The density of the gold particles that revealed the presence of
lipoxygenase in the cells of the nucellus was very low. Similarly to nucellus
cells of the young L. kaempferi ovule, single immunogold particles were found in the
cytoplasm and in the vacuoles of integument cells (Figs. 9 and 10). The density of the gold
particles that revealed the presence of lipoxygenase in the cells of the
integument was also similar to their density in the cells of the nucellus.
In a
megasporocyte (megaspore mother cell) of L. kaempferi, single immunogold particles were found in the
cytoplasm and in the area of the starch grain (Fig. 11). Single immunogold
particles were also localized in the vacuole and the cytoplasm in the vicinity
of cell organelles such as mitochondria (Figs. 12 and 13). Sometimes, in the
vacuoles, immunogold particles occured in pairs. In the megaspore mother cell,
immunogold particles were found in the area of the cell wall (Fig. 14). The
density of the gold particles that revealed the presence of lipoxygenase in the
cells of the megaspore mother cell was similar to their density in the cells of
the nucellus and the integument.
In
order to determine the degree of specificity of the immunogold reaction, a
control reaction including all the procedures was carried out. The control
reaction was conducted omitting incubation with the primary antibody. Only rare
gold particles (a few per one nickel grid) were found in the specimens. In most
grid meshes no gold particles were present (an example is shown in Figure 15).
DISCUSSION
The localization of the enzyme lipoxygenase (LOX; EC 1.13.11.12),
presented in this paper, was investigated in the cells of the ovule of Japanese
larch Larix kaempferi (Lamb.) Carr.. The application of the immunogold
labelling technique alowed us to precisely localize LOX on the cytological
level in the electron microscope. This method (i.e. the immunogold LOX PAb localization method) had been used earlier
for the localization of LOX on the cytological level in the different cells of
angiosperm plants. For example, the
occurrence of lipoxygenase in different parts and types of anther cells in Gagea lutea had been revealed with this
method (Szczuka et al., 2004, 2006, 2007, 2008).
Scientists,
mainly physiologists, have shown the occurrence of LOX in plants using various
methods. For example, the simplest way of LOX localization is determination of
the enzyme activity in individual plant parts by using the spectrophotometric
method. Apart from that technique, the enzyme activity in a plant extract can
be measured using (i) methods based on oxygen uptake (manometric or
polarographic techniques), (ii) methods based on formation of conjugated diens,
or (iii) determination of hydroperoxides (Grossman and Zakut, 1979).
Another
method used in the investigations concerning determination of lipoxygenase
isoenzymes is electrophoresis (SDS-PAGE, native PAGE, IEF) (Grossman and Zakut,
1979; Heinisch et al., 1996;
Smith et al., 1997).
Additionally, LOX activity can be determined by distinguishing between
lipoxygenase and heme proteins. Cyanide was suggested as a selective inhibitor
for distinguishing between them in the oxidation of fatty acids, but, according
to Grossman and Zakut (1979) lipoxygenase activity is also sensitive to
cyanide. These authors cited another method of distinguishing the activities of
heme and non-heme proteins, which is based on the different effects of
linoleate on the fluorescence of these catalysts.
The ovules of L. kaempferi investigated in this paper
were at an early stage of development with the megaspore mother cell in the
nucellus. Immunogold particles which revealed the presence of lipoxygenase were
found in all parts of the investigated young ovules – the integument, the
nucellus, and the megaspore mother
cell. Moreover, it was clearly visible that in the particular investigated
parts of the ovule the intensity of reaction was similar. In the young ovule cells of the nucellus, integument, and
megaspore mother cell, the density of the gold particles was very low. In case
of the investigations carried out in the presented in this paper, accurate
counting of the immunogold particles was immpossible to perform. Such a task
had been undertaken in the
investigations presented during a congress in Brasilia (Brazil) (Szczuka et
al., 2008). For this purpose, the computer program AnalySiS was used. The
obtained results concerned four different developmental stages of the L. kaempferi ovule and showed that in
the generative cells of the female line, the largest number of gold particles
labelling LOX occurred in megaspores, especially in the middle megaspore. The
smallest number was found in the egg cell. The immunogold particles occurred
most numerously in the integument cells. Especially large groups of immunogold
particles were found in the secretory cells of the integument. The smallest
number of immunogold particles was localized in the cells of the gametophyte.
The undifferentiated quantity of the immunogold particles in the particular
investigated parts of the ovule presented in this paper can be explained by the
very young stage of L. kaempferi
ovule development. At this stage, all parts of the ovule develop with similar
intensity. Therefore, the density of the gold particles was also similar. The
preprophase and the prophase periods of the first meiotic division in the
megaspore mother cell require more time than the other stages of
megasporogenesis. So, we can suppose that this is the main reason of the low
activity of lipoxygenase in the megasporocyte and the neighbouring cells.
The immunogold labelling method demonstrated that LOX occured the most often in two cell elements: the cytosol and vacuoles. In most cases, the immunogold particles were distributed randomly in both parts of the cells of the three investigated ovule parts: the integument, the nucellus, and the megaspore mother cell. The immunogold particles which revealed the presence of the enzyme were found to be associated in smaller quantities with membranes: the plasma membrane, short endoplasmic reticulum cisternae – mainly RER (rough endoplasmic reticulum), and the nuclear envelope. LOX was also detected near mitochondria and plastids. Some single immunogold particles were also observed at or in the area of the cell walls and the nuclei of the ovule cells. Similar observations concerning the immunolocalization of LOX in an electron microscope hed been described by several authors (for references see the introduction to this paper).
The immunolocalization of LOX with an
electron microscope indicated similarity in the distribution of lipoxygenase in
all cells of the particular investigated larch ovule parts to other, earlier
investigated, plant cells. However, the immunogold reaction in the cells of the
larch ovule was less intensive in comparison with other plant organs described
in the literature.
ACKNOWLEDGEMENTS
We would like to
thank the manufacturer for developing the antibody used in the experimental
part of this paper. Polyclonal antibody against antigen LOX was produced by Agrisera,
SE-911 21 Vännäs, Sweden, www.agrisera.se
LITERATURE
Bowsher C.G., Ferrie B.J.M., Ghosh S., Todd
J., Thompson J.E. and Rothstein S.J. 1992. Purification and partial
characterization of a membrane-associated lipoxygenase in tomato fruit. Plant Physiology, 100: 1802-1807.
Feussner I. and Wasternack C. 2002. The lipoxygenase
pathway. Annu. Rev. Plant Biol., 53: 275-297.
Feussner I., Hause B.,
Vörös K., Parthier B. and Wasternack C. 1995. Jasmonate-induced
lipoxygenase forms are localized in chloroplasts of barley leaves (Hordeum
vulgare cv Salome). Plant J., 7: 949-957.
Feussner I., Balkenhohl T.J., Porzel A., Kühn H.
and Wasternack C. 1997. Structural elucidation of oxygenated storage lipids in
cucumber cotyledons - implication of lipid body lipoxygenase in lipid
mobilization during germination. J. Biol.
Chem., 272: 21635-21641.
Grossman S. and Zakut R. 1979.
Determination of the activity of lipoxygenase (lipoxidase). Methods Biochem. Anal., 25: 303-329.
Heinisch O., Kowalski E., Ludwig H. and Tauscher
B. 1996. Staining for soybean lipoxygenase activity in electrophoretic gels. Fat/Lipids, 5: 183-184.
Lima de Carvalho W., Goreti de Almeida Oliveira M.,
Goncalves de Barros E. and Moreira, M.A. 1999. Lipoxygenases affect protease
inhibitor levels in soybean seeds. Plant
Physiol. Biochem., 37: 497-501.
Lynch D.V. and Thompson J.E.
1984. Lipoxygenase-mediated production of superoxide anion in senescing plant
tissue. FEBS Lett., 173: 251-254.
Rosahal S. 1996. Lipoxygenase in plants – their role
in development and stress response. Z. Naturforsch., 51c:
123-138.
Schmitt N.F. and van
Mechelen J.R. 1997. Expression of lipoxygenase isoenzymes in
developing barley grains. Plant Sci.,
128: 141-150.
Skórzyńska-Polit E., Pawlikowska-Pawlęga B., Szczuka
E., Drążkiewicz M. and Krupa Z. 2006. Localization and
activity of lipoxygenase in Arabidopsis thaliana plants under heavy
metal stress. Plant Growth Reg. 48: 29-39.
Skórzyńska-Polit
E., Pawlikowska-Pawlęga B., Szczuka E, Plak A. and Melke J. 2005. Localization
and activity of lipoxygenase in Cd-treated seedlings of Phaseolus coccineus.
Acta Soc. Bot. Pol. 3: 199-207.
Szczuka E., Skórzyńska-Polit E.,
Pawlikowska-Pawlęga B., Sobieska J. and Gawron A. 2004. Localization of
lipoxygenase in the anther of Gagea lutea. Materials of 18th
International Congress on Sexual Plant Reproduction. Beijing (China) 2004: 53.
Szczuka E., Skórzyńska-Polit E.,
Pawlikowska-Pawlęga B., Sobieska J. and Gawron A. 2006. Localization of
lipoxygenase in the anther of Gagea lutea. Acta Biol. Cracov., 48: 19-26.
Szczuka E., Seta A., Domaciuk M.,
Skórzyńska-Polit E., and Giełwanowska I. 2007. Lipoxygenase in the cells of
angiosperms. Materials of 3rd International Conference. Nauka i obrazowanie
bez granica. Biologia. Sofia 13:
9-18.
Szczuka E., Seta A., Domaciuk M., Skórzyńska-Polit
E., and Giełwanowska I. 2008.
Lipoxygenase in the cells of the developing ovule of Larix caempferi (Lamb.) Carr.
Materials of 20th International Congress on Sexual Plant
Reproduction. Brasilia (Brazil) 2008: 124.
Szczuka E. and
Skórzyńska-Polit E. 2008. Localization of lipoxygenases in higher plants
(in press).

LEGENDS
Fig. 1. General
habit of the Larix
kaempferi (Lamb.) Carr.
tree.
Fig. 2. L.
kaempferi. Female, old cones.
Fig. 3. Fragment of
the L. kaempferi trunk with bark characteristic of this species of
larch.
Fig. 4. L.
kaempferi. A piece of twig
with a female cone younger than shown in Figure 2, and with young generative
(one) and vegetative buds.
Fig. 5. A young
female cone of L. kaempferi.
Fig. 6. Bract and
seed scales of L. kaempferi.
Figs. 7 – 15. Immunolabelling to lipoxygenase in Larix
kaempferi.
Figs. 7 and 8.
Immunogold LOX PAb localization in the cells of the nucellus (at the stage of
megasporocyte in the nucellus) of L. kaempferi. Note single gold particles
(arrows) at the edge of the cytoplasm and vacuole (Fig. 7) and in the area of
the cell wall (Fig. 8). The arrow in Figure 8 points to a single particle at
the edge of the cell wall and cytoplasm. C –
cytoplasm, V – vacuole, CW – cell wall.
Figs. 9 and 10. A
portions of integument cells of the L. kaempferi ovule. Single immunogold particles in
the cytoplasm (C) and vacuoles (Fig. 9) and grouped immunogold particles in
vacuoles (Fig. 10). C – cytoplasm, V –
vacuole, CW – cell wall.
Figs. 11 – 15. A
portions of a megasporocyte (megaspore mother cell) of L.
kaempferi. Single immunogold particles (arrows) in the
cytoplasm (C), and in the area of the starch grain (S) (Fig. 11). Single
immunogold particles in the vacuole (Figs. 12 and 13), cytoplasm (arrow) (Fig.
13), and the cell wall (Fig. 14). M – mitochondrion, V – vacuole, CW – cell
wall.
Fig. 15. A portion
of a megaspore mother cell (megasporocyte). A control micrograph of the
cytoplasm (C) and the cell wall (CW).