The unique features and ultrastructure of the
microspore and pollen grain of the Antarctic dicotyledonous plant Colobanthus quitensis (Kunth) Barlt.
Irena Giełwanowska1, Ewa Szczuka2,
Irena Agnieszka Pidek3,
Aleksandra Seta2, Marcin
Domaciuk2
1* Department of Plant Physiology and
Biotechnology, University of Warmia and Mazury, Oczapowskiego 1A, 10-719
Olsztyn, Poland
** Department of Antarctic Biology, Polish
Academy of Science, Ustrzycka 10/12,
Warsaw, Poland
2 Department of Plant Anatomy and Cytology, Maria
Curie-Skłodowska University, Akademicka 19, 20-033 Lublin, Poland, eszczuka@biotop.umcs.lublin.pl
3 Department of Physical Geography and
Palaeogeography, Maria Curie- Skłodowska University, al. Kraśnicka 2
c/d, 20-718 Lublin, Poland
ABSTRACT
The
formation, structure and ultrastructure of microspore and pollen grain of Colobanthus quitensis (Kunth) Bartl.
(one dicotyledonous and one from the only two native flowering plants growing
in Antarctica) were investigated. Plant material was collected in the vicinity
of the Polish Antarctic Station. The studies of microspores and pollen grains
were carried out by means both light and electron microscopes.
The development of microspores and
pollen grains of C. quitensis takes
place in chasmogamic or cleistogamic flowers. Both the chasmogamic and
cleistogamic flowers of the investigated species usually form five stamina with
short filaments. One stamina contains two elongated microsporangia. The
microsporogenesis and male gametogenesis are carried out in the microsporangia
enveloped with a three-layer anther wall (epidermis, endothecium and tapetum).
In both processes microsporogenesis and male gametogenesis of the investigated
plant species proceed in a way typical of other angiosperms. C. quitensis forms spherical,
two-nuclear pollen grains enveloped by the thick polyporate sporoderm.
Characteristically, there is no callose wall between generative and vegetative
cells in the bicellular pollen grain.
INTRODUCTION
Colobanthus quitensis (Kunth) Bartl.
(Caryophyllaceae) is one of the only
two native flowering plants that grow in Antarctica and the only native
dicotyedonous plant on the continent. Antarctica is considered to be the most
distant, inhospitable, the coldest and the most inaccessible polar region of
the Earth (Giełwanowska and Szczuka, 2005; Giełwanowska et al., 2005;
Szczuka et al., 2007; Szczuka et al., 2008). The only possible period for
vegetation is the summer period which lasts no more than 5 months, typically
from November to March (in this case the length of day and night is taken into
consideration). Actually, the plant growth season is shortened to no more than
2-3 months, because of the length of the snow-cover period in the areas of
Antarctica that are uncovered by ice.
The
mentioned above and other climatic events that result from the severe
conditions of the Antarctic region make growing plants develop various
adaptation mechanisms. In order to survive, C.
quitensis, the Antarctic pearlwort have developed well functioning
physiological and biochemical adaptations to adapt to rapidly fluctuating
growth conditions. Proving this is the fact, that pearlwort flowers profusely
and produces seeds almost each year (Giełwanowska et al., 2007; Giełwanowska
et al., 2007). Additionally, unfortunately shy in number literature and data
concerned the investigations of flower and seed development both Antarctic
pearlwort and Antarctic hair grass is given in the paper of Giełwanowska
et al., (2007).
On the
other hand, the specificity of extremely severe climatic conditions of the
Antarctic region induce scientists to investigate the possibility of plant
growth and development in this area. C.
quitensis can be considered a good plant model for analysis of various adaptation mechanisms to the both biotic
and abiotic stress factors acting in Antarctica (Levi-Smith, 2003). The
knowledge of the biology concerning
flowering and reproduction of these species is still insufficient. Only
a few papers report the results of the investigations concerning the generative
male line development (Giełwanowska 2005; Giełwanowska et al., 2005
Giełwanowska et al., 2006. Therefore, the focus of this paper is on the
features and ultrastructure of microspores and pollen grains of Antarctic
flowering plant C. quitensis.
MATERIAL AND
METHODS
Plant material
Flower
buds of Colobanthus quitensis (Kunth)
Bartl. (Caryophyllaceae) at various stages of development were collected from
plants growing under natural conditions near H. Arctowski Antarctic Station
(62º09.8' S, 58º28.5' W) in the area of SSSI (Site of Special
Scientific Interest) No. 8. on King George Island (the South Shetland Islands)
(see map in Fig. 1). Fresh flower buds of C.
quitensis were picked up during the Antarctic summer, mainly in January
2004 during 26th expedition to the Antarctic, organized by the
Department of Antarctic Biology, Polish Academy of Sciences in Warsaw.
Light microscopy
Flower buds were fixed in 3.5%
glutaraldehyde in 0.1 M phosphate buffer (pH 7.0) for 8 hours at room
temperature. The fixed buds were washed in two changes of 0.1 M phosphate buffer and postfixed
overnight in 2.5% OsO4.
The material was then washed in buffer, dehydrated in a graded ethanol series,
and transferred into mixtures with increasing ratios of Poly Bed 812 resin.
Semi-thin sections (1-2 μm thick) were stained with toluidine blue and
observed under the light microscope.
Electron microscopy
For transmission electron microscopy (TEM), small pieces of sporangia
were fixed in 2% glutaraldehyde in 0.05 M sodium cacodylate buffer (pH 7.2).
Specimens were washed three times in 0.05 M sodium cacodylate buffer and
post-fixed in 1% osmium tetroxide. Samples were dehydrated in graded ethanol and aceton series followed by
propylene oxide, infiltrated and embeded in Spurr’s resin (Spurr, 1969). The
material was sectioned at 50 nm and stained with 2% uranyl acetate and lead
citrate (Reynolds, 1963), for 30 min.
Finally, thin sections were studied with the JEOL JEM 100S transmission
electron microscope.
RESULTS
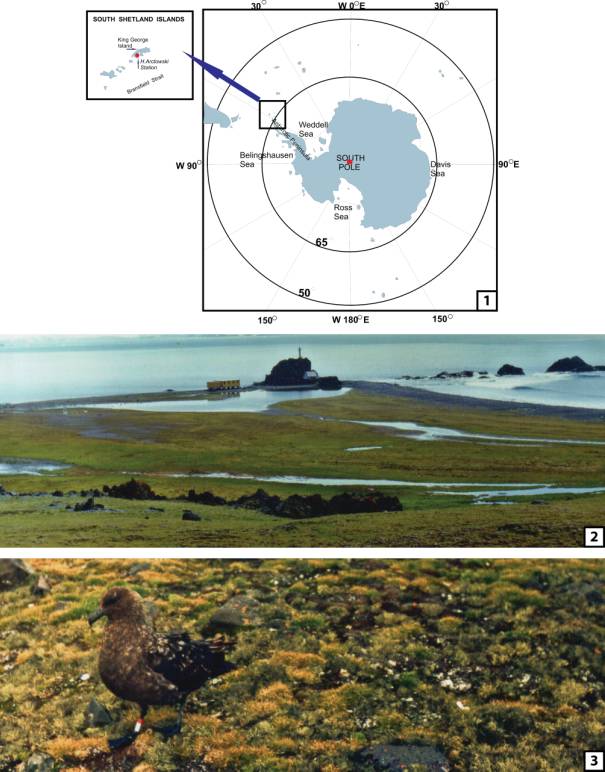
The Colobanthus quitensis is a small
dicotyledonous plant. In the investigated area (vicinity of Henryk Arctowski
Polish Antarctic Station on King George Island) (Fig. 2), the plants usually
grow next to the Deschampsia antarctica (Monocotyledones), mosses and lichens,
forming flat, dense mats (Fig. 3).
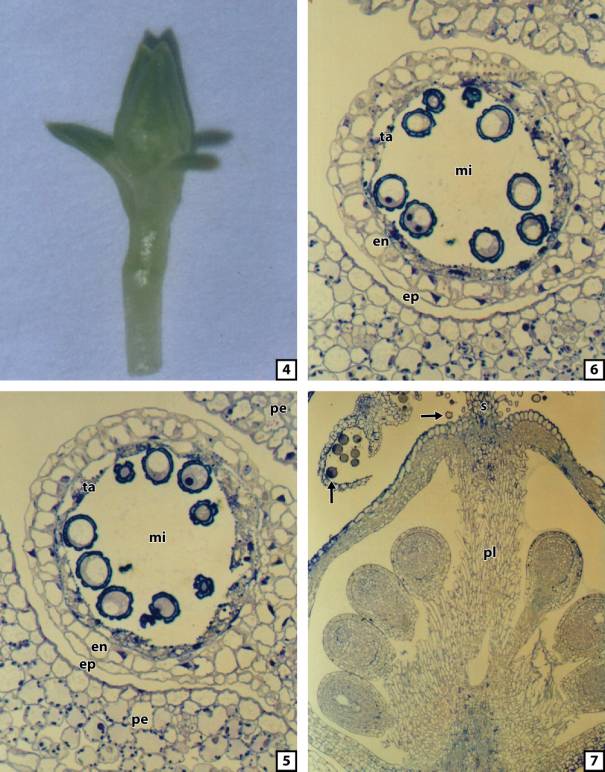
In natural conditions, C. quitensis, a hardy, perennial plant,
forms cushions consisting of numerous multi-module individuals. The single,
small, inconspicuous, and bisexual buds or flowers (Fig. 4) of this species may terminate module shoot or may be
axillary. In the investigated species C. quitensis varies from
the cleistogamic (closed) flowers typically formed in unfavorable conditions.
In the case of the latter, the pollen grains are scatterred after anthesis in
the vicinity of D. antarctica
tussocks or C. quitensis tufts. In
the investigated Anatarctic pearlwort, the stamens (usually 5) and the pistil
coated with five elements of the undifferentiated perianth develop in closed
flower buds hidden between the leaf
blades. The number of perianth elements may vary, from four to six of them.
Green sepals arranged in two verticils form the perianth.
The central part of the young
microsporangium is occupied by archesporial tissue, as well as microsporocytes.
After meiosis with simultaneous cytokinesis, tetrahedrally arranged microspores
(tetrads of microspores) are formed from microsporocytes. The microspores
released from the tetrad are enveloped by the sporoderm. Single microsporangium
is circular in a transverse section. At the stage when microspores are in the
loculus, the external layer (epidermis) envelops a single cell layer of
endothecium, cellular tapetum, and microspores (Fig. 5).
After mitotic division of the
microspores, two-nuclear pollen grains appear in the loculus. At the time when
the pollen grains are formed the structure of the single microsporangium does
not change in any significant way. During the previous stage of development
with microspores in the loculus, the microsporangium in a transverse section is
regular and circular in shape.
Similarly to the previous stage, at the stage when pollen grains are in
the anther loculus, the wall of the microsporangium is built out of three
layers of cells: the external – epidermis, middle – endothecium, and the most
internal – tapetum (Fig. 6).
A
longitudinal section of a closed flower of C.
quitensis shows its detailed anatomical structure with the ovules settled
on the placenta and pollen grains in opened microsporangia or released from
microsporangia (Fig. 7). Mature pollen grains of C. quitensis are rather spherical, two-nuclear, and enveloped by
the polyporate sporoderm. A
longitudinal section of a closed flower of C.
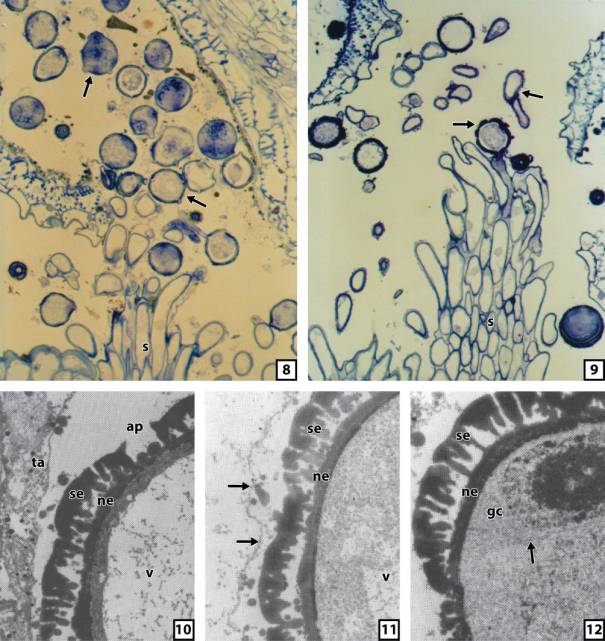
quitensis allows observation of the germinating pollen grains in opened
microsporangia or released from microsporangia. Even the very early stage of
pollen grain germination shows two or more pollen tubes growing from a single
pollen grain (Fig. 8). Nevertheless, on a dry, pulmose stigma only one pollen
tube from single pollen grain wins the competition (Fig. 9).
The
ultrastructural details of the microspore and pollen grain structure with
special attention paid to the sporoderm are presented in the figures ten to
twelve. The complex cell wall, at first sporoderm is formed on the surface of
the microspore protoplast. Nevertheless, its structure is similar to the
sporoderm that envelopes mature pollen grains (Figs. 10 and 11). In both cases
of the microspore sporoderm and the pollen grain sporoderm consist of electron-dense
sporopollenin material which is not present in apertures. In most pollen
grains, the sporoderm is perforated in about a dozen apertural sites. The
separating callose wall between generative and vegetative cells of pollen grain
is not visible during observation through the
TEM (transmission electron microscope) (Fig. 12).
DISCUSSION
Microsporogenesis
of the investigated plant species Colobanthus
quitensis proceeds in a way typical of other angiosperms. In C. quitensis,
microsporogenesis ends with simultaneous cytokinesis. Simultaneous cytokinesis
is often meet in dicotyledonus plants. The other type of cytokinesis -
successive cytokinesis is characteristic of monocotyledones. As in the other
angiosperms, the microspores at the tetrad stage of C. quitensis are surrounded with a thick callose wall (data not
shown in any figure in this paper). This callosic wall accompanies the dividing
microsporocyte (microspore mother cell) from the earlier first meiotic
prophase. During meiosis the callose wall becomes thicker and fulfill the
isolational functions until the tetrad stage begins (Giełwanowska at al.,
2007). Callose begins to hydrolise when the construction of sporoderm starts (Heslop-Harrison et al., 1986).
The sporoderm enveloping the microspore
and, later, the pollen grain of C.
quitensis is relatively thin. The osmophilic material, in the form of
proorbicules, moved from various areas of the tapetal cells towards pollen
grains, and in the form of orbicules joined the developing sporoderm. The formation
of the sporoderm is similar to this process of the other pollen grain sporoderm
in different plant species (Raghavan, 2000).
Similarly
to microsporogenesis, male gametogenesis is typical of other angiosperms (for
detailed description of both processes in flowering plants see Raghavan, 2000).
Characteristically, in C. quitensis,
the separating calose wall is not formed between generative and vegetative
cells. The existence of a cell wall separating the generative cell from the
vegetative cell has been controversial. No doubt, that the generative cell is
usually bound by the plasma membrane and a distinct cell wall. The cell wall
often contains callose, e.g. in orchids (Heslop-Harrison, 1968a). The
generative cell of Anatarctic pearlwort, immersed in the cytoplasm of the
vegetative cell, was observed through a transmission electron microscope
(Giełwanowska et al., 2007). Using the same technique, the male germ unit
of C. quitensis was investigated. The
morphological features of the generative cell and sperm cells of Anatarctic
pearlwort are comparable to the features of
cells in Plumabago zeylanica
(Russel, 1984) or Brassica (Dumas et
al., 1985; Charzyńska et al., 1989)
In
closed flowers of C. quitensis microsporangia
dehisce releasing pollen grains, which reach the stigma surface and germinate
(Giełwanowska et al., 2005; Giełwanowska et al., 2005; Szczuka et
al., 2006). The pollen grains of C.
quitensis can germinate inside the closed microsporangium. Often pollen
grains of this species germinate with two or more pollen tubes.
REFERENCES
Charzyńska M., Murgia M.,
Milanesi C., Cresti M., 1989. Origin of sperm, cell association in the “male
germ unit” of Brassica pollen. Protoplasma, 149: 1-4.
Dumas C., Knox R.B., Gaude T.,
1985. The spatial association of the sperm cells and vegetaive nucleus in the
pollen grain of Brassica. Protoplasma
124: 168-174.
Giełwanowska I., 2005. Specyfika rozwoju antarktycznych
roślin naczyniowych Colobanthus quitensis (Kunth) Bartl. i Deschampsia antarctica Desv.: 21-65,
Wydawnictwa UWM, Olsztyn.
Giełwanowska I., Bochenek A., Loro P., 2005. Biology of
generative reproduction of Deschampsia
antarctica: 181-195, L. Frey (Ed.), W. Szafer Institute of Botany, Polish Academy
of Sciences, Kraków.
Giełwanowska I., Szczuka E., Bednara J., Górecki R., 2005. Anatomical features
and ultrastructure of Deschampsia
antarctica (Poaceae) leaves from different growing habitats. Annals
of Botany 96: 1109-1119.
Giełwanowska I., Szczuka
E., 2005. New ultrastrucural features
of organelles in leaf cells of Deschampsia antarctica Desv. Polar Biology 28:
951-955. DOI
10.1007/s00300-005-0024-2.
Giełwanowska I., Szczuka E., Bochenek A., 2005. Zapylenie u
antarktycznej rośliny kwiatowej Colobanthus quitensis (Kunth)
Bartl. Materials
of V Polish Scientific Conference entitled „Biology of flowering and pollen
alergy” (in Polish), Lublin, 25.
Giełwanowska I., Szczuka E., Bochenek A., 2006. Zapylenie u
antarktycznej rośliny kwiatowej Colobanthus quitensis (Kunth)
Bartl. Acta
Agrobotanica 59(1): 123-131.
Giełwanowska I., Bochenek A., Szczuka E., 2007. Development of the
pollen in the Anatarctic flowering plant Colobanthus quitensis (Kunth)
Bartl. Acta Agrobotanica Vol. 60(2): 3-8.
Giełwanowska I., Bochenek A., Szczuka E., 2007. Development of the
pollen in the Anatarctic flowering plant Colobanthus quitensis (Kunth)
Bartl. Materials of VI Polish Scientific Conference entitled „Biology of
flowering and pollen alergy” (in Polish),
Lublin, 36.
Heslop-Harrison J., 1968a. Synchronous pollen mitosis and the formation of
the generative cell in massulate orchids. J. Cell Sci. 3: 457-466.
Heslop-Harrison J.,
Heslop-Harrison J.S., Heslop-Harrison Y., 1986. The compartment of the
vegetative cell in the pollen and pollen tubes of Helleborus foetidus L. Ann. Bot. 58: 1-12.
Levis-Smith R.I., 2003. The
enigma of Colobanthus quitensis and Deschampsia antarctica in Antarctica. In
: Antarctic Biology in a Global Contekst. Eds. Huickes A.H.I., Gieskes W.W.C.,
Schorno R.L.M., van der Vies S.M. and Volff W.I., pp. 234-239. Backham
Publishers, Leiden, The Netherlands.
Raghavan V., 2000.
Developmental biology of flowering plants, Springer-Verlag New York, Inc. pp.
186-227.
Reynolds E.S., 1963. The use of lead citrate of high pH as an electron -
opaque stain in electron microscopy. J. Cell Biol. 17: 208-212.
Russel S.D., 1984. Ultrastrucure of the sperm of Plumabago zeylanica II. Quantitative cytology and three-dimentiolan
organization. Planta 162: 385-391.
Spurr, A.R., 1969. A low viscosity epoxy resin embedding medium for
electron microscopy. J. Ultrastruct. Res. 26: 31-43.
Szczuka E.,
Giełwanowska I., Seta A., Domaciuk M., 2006. Pollen and pollination in the
Antarctic flowering plant of Colobanthus
quitensis (Kunth.) Bartl. Materials of XIX th International
Congress on Sexual Plant Reproduction
“From gametes to genes”. Budapest, Hungary, 11-15 July 2006. p. 221.
Szczuka E.,
Giełwanowska I., Pidek I.A., Seta A., Domaciuk M., 2007. Pollen of the
Antarctic plants Colobanthus quitensis
and Deschampsia antarctica and its
representation in moss polsters. Materials of 6 th International
Meeting “Pollen Monitoring Programme”. Jurmala, Latvia, 3-9 June 2007. p.
78-80.
Szczuka E.,
Giełwanowska I., Pidek I.A., Seta A., Domaciuk M., 2008. Pollen of the
Antarctic plants Colobanthus quitensis
and Deschampsia antarctica and its
representation in moss polsters. in press
LEGENDS
Fig. 1. Location map of King
George Island in the South Shetland Islands Archipelago and Cockburn and
Seymour Islands in the Antarctic Peninsula sector. Arrow shows the magnified
area of main map.
Fig. 2. The vicinity of the
Polish Polar Station on King George Island.
Fig. 3. Colobanthus quitensis and Deschampsia
antarctica in a natural habitat in the vicinity of the Polish Polar Station
on King George Island. Both species grow here together with mosses, and form
flat, dense mats.
Fig. 4. Flower bud of Colobanthus quitensis.
Fig. 5. A magnified
microsporangium (mi) of Colobanthus
quitensis with microspores in the loculus surrounded with sepals (pe). A
semi-thin, transverse section stained with toluidine blue. Visible epidermis
(ep), endothecium (en) and disintegrated tapetum (ta). 2000 x.
Fig. 6. A magnified
microsporangium (mi) of Colobanthus
quitensis with pollen grains in the loculus . A semi-thin, transverse
section stained with toluidine blue. Visible epidermis (ep), endothecium (en)
and disintegrated tapetum (ta). 2000 x.
Fig. 7. A longitudinal section
of a closed flower of Colobanthus
quitensis with ovules settled on placenta (pl) and pollen grains in opened
microsporangium (left arrow) or released from microsporangia. Pollen grains
(arrow) on a dry, pulmose stigmas. A semi-thin section stained with toluidine
blue. 1000x
Fig. 8. A longitudinal section
of a closed flower of Colobanthus
quitensis with germinating pollen grains in opened microsporangia or
released from microsporangia. Early stage of pollen grain germination. Pollen
grains (arrows) on dry, pulmose stigma.
A semi-thin section stained with toluidine blue. 2500 x.
Fig. 9. A longitudinal section
of a closed flower of Colobanthus
quitensis with germinating pollen grains released from microsporangia. Later than in Figure eight stage of pollen
grain germination. Pollen grains (arrows) on dry, pulmose stigma. A
semi-thin section stained with toluidine blue. 2500 x.
Fig. 10. TEM. The ultrastructure
of the microspore sporoderm of Colobanthus quitensis and neighbouring
tissue of the microsporangium wall. Note disintegrated tapetum (ta). ap –
aperture, ne – nexine, se – sexine, v – vacuole. 20000 x.
Fig. 11. TEM. The
ultrastructure of the pollen grain sporoderm
of Colobanthus quitensis. Note
disintegrated tapetum with tapetal membrane (arrows). ne – nexine, se – sexine,
v – vacuole. 20000 x.
Fig. 12. TEM. The
ultrastructure of the pollen grain of Colobanthus
quitensis. Arrow points the cell wall separating generative and vegetative
cells. gc – generative cell, ne – nexine, se – sexine. 20000 x.