Infrared spectroscopy for
determination degradation of polymers
V. PETRÁnek1,
J. Kosíková2,
1
Brno University of Technology,
Faculty of Civil Engineering, Institute of Technology of Building Materials and
Components, Brno, Czech Republic, Tel.: +420 541 147 511, petranek.v@fce.vutbr.cz
2
Brno University of Technology,
Faculty of Civil Engineering, Institute of Technology of Building Materials and
Components, Brno, Czech Republic, Tel.: +420 541 147 521, kosikova.j@fce.vutbr.cz
1.
Abstract:
Infrared
spectroscopy method is very extended in various branches. The possibility of
using infrared spectroscopy in civil engineering is described in this paper. At
first, there is description of principals of infrared spectroscopy method,
preparation of samples and common utilization of IR. In the end of
paper the possibilities of determination of degradation of polymers by mentioned
method and as example the beginning of PVC degradation experiment is described.
The experiment is still in progress and will continue.
2. Introduction:
Infrared spectroscopy (Infra Red,
IR)
is one of the most significant analytic technologies – it is known since 1940,
however this technique became widespread and broadly used in
chemical-technological branches just in last 20 years because of availability
of more powerful computers. Also in building industry have IR spectroscopy
found its usage in few recent years.
The advantage of IR spectroscopy is the possibility to
study any type of a sample through liquids, solutions, pastes, powders, films,
fibres, gases and surfaces of different materials and also in little amount.
IR spectroscopy as physical technique of measurement is based on the interaction
between infrared thermal radiation with the studied matter.
Principle of IR spectroscopy:
Principle of measurement technique is in measuring of
absorbed IR radiation of different wavelengths, which passes through analyzed
material. It is based on vibrations of atoms in a molecule. The intensity
increases if the IR radiation is absorbed by the material. Each chemical bond
requires exact amount of energy to start to vibrate.
It is about electromagnetic radiation that occurs in
an area of wavelengths of 0.78 - 1 000 mm (it is 12 800 - 10 cm-1).
Generally we distinguish IR radiation among near (13 000 - 4 000cm-1),
middle (4 000 - 200 cm-1) and distant (200 - 10 cm-1).
Principle of the method depends on the absorption of IR radiation while passing
through the sample, during the absorption occur changes of vibration-rotation
energetic states of the
molecule in relation on changes of dipole moment of the molecule.
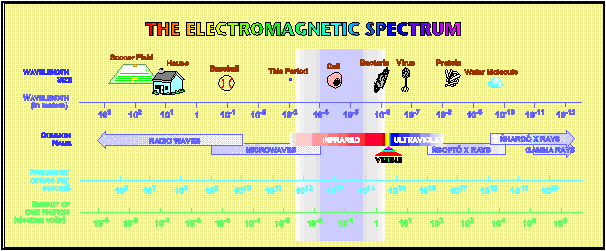
Fig 1: Electromagnetic spectrum
Analytical output is IR spectrum, which is graphical display
of functional dependence of the energy, often expressed in percentage of the
transmittance (T) or in units of absorbance (A) on the wavelength of incident
radiation. The transmittance is defined as the ratio of intensity of radiation
that passed through the sample (I) and the intensity of radiation emitted by
the source (Io). Absorbance is defined as the common logarithm 1/T. Wave number
is defined as inverted value of wavelength.
Preparation of samples:
As
I have already mentioned, this method can be used to measure samples in all
states of matter and in different modifications.
Liquid samples are being
measured as pure fluid or solutions. For the measurement we use liquid cuvette
consisting from two plan-parallel plates from NaCl or KBr. Also we can form
thin layer between two plates or we can use polymer porous carrier.
For measurement of
gaseous matter, special gaseous cuvette with front walls made from material
transmitting IR radiation, is used. The plates are in most cases made from NaCl
a KBr. Cuvettes are often 10cm long. We can also use so called „light pipe“
having small volume.
Samples
in solid form absorb IR radiation intensely so it is not so easy form from
these matters such thin layer that would transmit IR radiation. Because of this
we use these three techniques: KBr tablets – made from homogenized mixture of
the powder from the sample and KBr. Homogenization often takes place in special
vibrating mill, in steel homogenization capsule with little ball. Next the
mixture undergoes process of pressing, with maximal pressure of 80KN (in
vacuum) in steel bottom die under decreased pressure, into 0,5mm thick tablet.
This tablet is fastened into special holder and is installed into sampling
area. Because the fact that KBr is very hydroscopic, it has to be dried before
usage and the tablet is measured immediately after creation. KBr does not show
any absorption of middle IR area.
Nujols method – is suitable just for qualitative measurement. Nujol mixture is formed
by mixing of powder of a crushed sample and a drop of paraffin oil. This
mixture is prepared by attrition in agate bowl or among two straight vitreous
surfaces, which have to be sharpened till the last phase before polishing. IR
spectrums of suspensions are often very quality.
Films – develop after
mixing of sample with volatile dissolvent and deposition of this mixture on
NaCl plate. After evaporation of dissolvent reminds on the plate thin film,
that is carefully peeled away and is measured separately. This technique is
most suitable for polymers.
Common usage of IR
With the help of vibrating IR spectroscopy it is possible to study many
chemical and physical properties of samples. For example: changes in the
structure of molecules, isomerisation, polymerization, relative interaction of
molecules, chemical reactions, phase transition, dissolvent effect, adsorption
of molecules on the surface. This method is plentifully used in criminal
science for identification of toxic and addictive matters, in pharmaceutical
and in food processing industries. IR spectroscopy has special applications in
case of studying polymers. It is mainly about identification of polymer
material, about qualitative and also quantitative determination of its chemical
composition (stating final groups, branching of chains, configurations and
conformations and so on), also to determinate concentration of impurities,
antioxidants, additives and emulsifiers, softeners, fillings, and residual
monomers. With respect to slowness of processes it is also possible observe
such processes as vulcanization, polymerization or degradation. Last but not least it is as well possible to
study the influence of outer conditions on polymers (temperature and pressure,
radiation, deformation, influence of aging or humidity of surrounding environs)
such as on other building material.
For the measurement exist several types of
instruments, working on different principles, however the most significant area
of IR spectroscopy is usage of Fourier transformation. Spectrometers working on
a principle of this transformation have higher quality of spectrum and require
less time to get data.


Fig. 2: Infrared spectometer working on principle
of Fourier transformation
As an example of usage IR spectroscopy in connection
with degradation of plastic matters, I would like to mention polyvinyl
chloride, which I concern as the most widespread plastic material in building
industry. By the method of IR spectroscopy were observed chemical changes
caused by the influence of UV radiation.
3. Determination of degradation of
polymers
As
a degradation of plastic matters we identify negative changes of their
properties or structure, which are caused by outer conditions and lead to
devaluation of material. There is a huge amount of outer conditions that
influences polymers in this way. The most common are high temperature, solar
radiation, water, humidity, biological agents, chemical activity and mechanical
charge. Main part of these conditions causes change of chemical structure of
polymers, which we can easily reveal by IR spectroscopy. For clearness I have
picked the case of degradation PVC under the influence of UV radiation.
PVC
Polyvinyl chloride (PVC) is the most significant representative of vinyl polymer group.
Together with PE and PP is the most commonly produced synthesis polymer. The
reasons for the expansion of PVC are cheap production processes and important
properties. PVC is relatively very resistant to the influence of aggressive
agents and organic dissolvent with non-polar character, but it underlie thermal
degradation (even from 100°C) and degradation of light very easily.
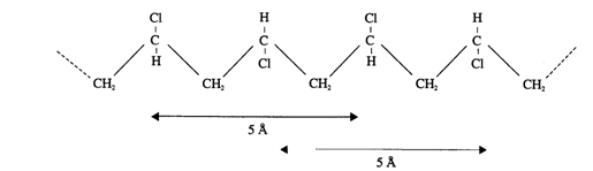
Fig.3: Structure PVC – PVC contains 56% of chlorine.
It forms chains, which are partly direct and partly branched. Also presence of
few double bonds is supposed. The structure is often syndiotactic.
Its elaboration, on hard or softened material requires
additives of different matters, which can influence resistivity of final
material against atmospheric aging. Structure of additives and admixtures
differs, in case of different processors, in quality and also quantity
Degradation of PVC
The reason for investigation is eg. the fact that it
is used as roof hydro-isolation and sometimes the degradation of the material
is relatively very quick.
This method (nearly non-destructive) may even on the
small piece of material prove finishing persistence of the material.
If we focus on degradation caused by the solar radiation we talk about
photo degradation. The source of this radiation influencing degradation
processes is the Sun. Solar radiation acting on irradiated plastic may be by
its surface reflected, dispersed, transmitted or absorbed. This radiation cause
changes in appearance, extents, but mainly chemical changes, with which relate
changes of mechanical properties and so distinct decrease of practical
persistence of the material. Photochemical changes take place, only if solar
radiation of certain wavelength is absorbed.
Generally is true, that for example carbonyl group C=O
absorbs radiation with the wavelength 187 nm and in range of length from 280 nm
to 320 nm. Bond C-C absorbs radiation with the wavelength 195 nm and 230 nm up
to 250 nm. Plastics containing these groups, while being radiated by these
wavelengths, will induce photochemical reactions. By the absorption of solar
radiation will increase volume of energy of the molecule, which gets to the
higher energy state. The biggest part of absorbed energy is depleted for the
transmission of the electron system to the higher quantum state. The rest of
the energy is used for the formation of free radicals in macromolecules
indicating the decay of some plastics.
The main chemical reaction taking place during solar
radiation action but also because of other atmospheric factors is splitting of
hydrogen chloride from the macromolecule of polymer PVC.
Groups reactive within PVC are mainly atoms of
chloride and unsaturated bonds, which develop by the transmission of monomer,
by finishing of disproporcionations and last but not least during thermal
processes. The effect of aerial oxygen form carbonyl groups as well. PVC
belongs among polymers, which under the influence of temperature split of low
molecular products, so called destruct. The level of destruction is dependent
on the structure of polymer, on the intensity of radiation, on the wavelength
of radiation and on surrounding conditions. Destructive waste product is HCl,
which may in humid environment very intensely corrode the manufacturing
instruments. With progressing destruction, PVC turns through yellow, orange and
brown into black colour. In case of splitting of cca 4 % HCl it becomes insoluble.
We can identify double bonds and carbonyl groups mentioned above with the help
of IR spectroscopy.
4. Practical determination /
experimental measurement?
For the case of measurement with the help of IR
spectroscopy was used sample of roof hydro-isolation from PVC – Fatrafol 804.
For determination of influence of UV radiation on this material was used rapid
aging in apparatus Solarbox, where is in laboratory conditions solar radiation
substituted by UV radiation. The sample was exposed UV radiation in testing
apparatus Erichsen Solarbox 522/1500. As a source of UV radiation is there used
xenon lamp, wavelength of the apparatus was 550 nm. Output of the lamp is
800W/m2 and in the room is also higher temperature (app. 55°C). Duration of exposition of experimental sample
was 1000 hours. The sample was measured after 500 hours and after 1000hours
again, resulting spectra were compared with spectrum measured before the
experiment.
The apparatus on which the samples were measured is
Nicolet 380, which is easily serviceable and for upkeep undemanding ant it make
FTIR spectroscopy accessible to all routine users. The apparatus is controlled
by computer through software OMNIC. We use this apparatus in our development
laboratory also for measurement of samples of our clients.
Comparison of spectrums is evident from the next
figure. The experiment is still running and samples are with respect to
non-destructive character exposed to UV radiation and regularly tested after
500 hours.
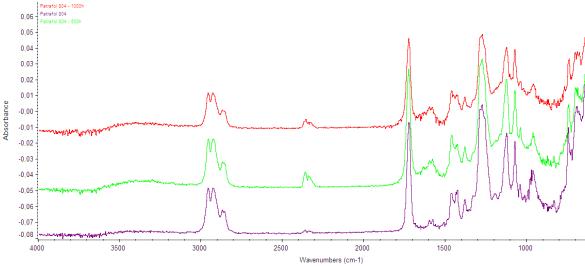
Fig. 4.: Spektrum PVC - before experiment, afther 500 and 1000hod in Solarbox
From this figure is evident, that there
are not observed any changes in chemical structure so I consider that after
1000 hours degradation of this hydro-isolation does not take place. Belts from
2950 to 2930 cm-1 are elastic bonds -CH2, belts 1725cm-1
are C═O bonds. Peaks 2360cm-1 are peaks of aerial CO2 and
they develop under the influence of environment in the laboratory, in the
surrounding area of 1100 – 1030cm-1 occur bonds Cl-C. In case that we want prove that this
material degrade, we have to choose probably much longer duration of UV
radiation exposition.
5. Conclusion
Finally we can say that the method of IR spectroscopy, working on the principle
of measuring of absorbed electromagnetic radiation by the material is suitable
for measurements in all physical states. It is very easy and quick method and
so that is why it is used in different fields of specialization, for example:
criminal science, pharmaceutical and food processing industries and in last 20
years it becomes important also in building industry. It is very convenient in studying chemical structure and
for determination of concentration of impurities, additives and emulsifiers,
softeners, fillings, and residual monomers. We can also observe with the help
of IR spectroscopy such processes as vulcanization and polymerization. Last but not least it is as well possible to
study the influence of outer conditions on polymers and degradation of material
caused by these conditions.
This method of IR spectroscopy was applied
to study degradation of roof hydro-isolation film made from PVC, caused by the
influence of UV radiation. With respect to intensity of radiation in Solarbox
was supposed that material will reveal HCl. By temporary measurement made on
samples was discovered, that even after 1000 hours of irradiation by UV
radiation in Solarbox, the material stays unchanged. This material is still
being exposed to radiation and we regularly test its chemical changes.
6. Acknowledgment
This
paper was prepared with financial support from the research project CEZ - MSM
0021630511, entitled: “Progressive Building Materials with Utilization of
Secondary Raw Materials and their Impact on Structures Durability”.