I.
Pronin*, I. Averin*, N. Kaneva, S. Siuleiman,
A.Karmanov*, L. Krasteva, A. Bojinova, K. Papazova, D. Dimitrov, S. Igoshina*
Laboratory of Nanoparticle Science and Technology,
Department of General and Inorganic Chemistry, Faculty of Chemistry and Pharmacy,
University of Sofia, Sofia 1164, Bulgaria
* Penza State University, Russia
Pharmaceutical drugs photodegradation by nanosized ZnO films
1.
Introduction
Nanostructured materials offer promising
opportunities for improved and tailored properties for application in
environmental catalysis due to their unique physicochemical properties, caused
by their nanosized dimensions and large surface/volume ratios.
Currently, ZnO semiconductor has stimulated
great research interest due to its unique optical and electrical properties
(nanolasers, piezoelectric nanogenerators, solar cells, gas sensors, good
stability, considerable application in photocatalytic reactions in the process
of elimination of water contaminants (dyes, pharmaceutical drugs). Several
works report the synthesis and high photocatalytic efficiencies of ZnO
nanoparticles, powders and colloids. But for water treatment applications, ZnO
thin films are preferred to avoid the separation of the catalyst after the
degradation process. Several works have been published in respect to
photocatalytic properties of ZnO thin films prepared by different methods - pulsed
laser deposition (PLD), chemical vapour deposition (CVD), magnetron sputtering and sol–gel. The sol–gel method has been
receiving high attention since it enables us to develop low-cost and simple
deposition procedure to obtain large area high quality ZnO films for
technological applications.
This paper describes kinetic studies on the
photocatalytic degradation of pharmaceutical drugs (Paracetamol and
Chloramphenicol) by ZnO thin films annealed at different temperatures. The
semiconductor photocatalysts are prepared vie sol-gel method using dip coating
technique. Microstructure, surface morphology and photocatalytic properties of
the nanostructure films are explored and discussed.
2.
Experimental
ZnO thin films were prepared by the sol-gel method
from zinc acetate dehydrate (≥99.5 %), 2-methoxyethanol (≥99.5%),
monoethanolamine (≥99.0%);
all of them from Fluka. Zinc acetate dehydrate was dissolved in a mixture
of 2-methoxyethanol and monoethanolamine. The mixture solution was stirred at 60
oC for 1 h to yield a clear and homogeneous solution. After the
solution was made 1 day at room temperature (23±2 oC), it was coated
on glass substrates (ca. 76 x 26 mm, from ISO-LAB (Germany)), using dip coating
technique (0.9 cm/min). Then
precursor thin films were heated at 80 °C for 15 min to remove the solvent and organic
residuals. The coating and heating process was repeated for 5 times. Then the
as-prepared thin films were inserted to a furnace and annealed in ambient
atmosphere at several temperatures from 100, 300 and 500 oC for 60
min.
Surface morphology
of the thin films deposited on glass substrate was measured by Scanning Electron
Microscopy (SEM, JSM-5510 (JEOL)). The crystallization behavior of the ZnO thin
films deposited was analyzed by X-ray diffractometer (XRD, Siemens D500 with
CuKα radiation within 2θ range 30-70 deg at a step of 0.05 deg
2θ and counting time 2 s/step). The average crystallite sizes were
estimated according to the Scherrer’s equation.
The pharmaceutical drugs – Paracetamol (C8H9NO2,
Actavis) and Chloramphenicol (C11H12Cl2N2O5,
Actavis), widely used products, were employed as a representative analgesic and
antibiotic to evaluate the photocatalytic activity of ZnO thin films. The
experiment was performed in a 150 ml glass reactor, equipped with magnetic stirrer (rotating speed ~ 500
rpm controlled by stroboscope) and UV-lamp (Sylvania BLB, 315-400 nm of
emission range, 18 W). The light power density at the sample position was 0,66
mW/cm2 as measured with Research Radiometer of Ealing Electro-optics,
Inc.). The distance between the sample and the high pressure mercury lamp was
15 cm. The mineralization of the PCA and CA solutions were measured at
intervals of 1h and the total irradiation time is 4 h by UV-VIS absorbance
spectroscopy (spectrophotometer Evolution 300 Thermo Scientific, wavelength range from 200 to 400 nm). The extent of
photocatalytic degradation could be evaluated by measuring the absorbance of
the solutions at 243 and 278 nm. The degradation efficiency of PCA and CA were
calculated using the equation:
Degradation%=(C0-C)/C0*100 = (A0-A)/A0*100 (1),
where C0 represents the initial concentration, Ct represents the
concentration after t min reaction, A0 represents the initial
absorbance, and At
represents the absorbance after t min
reaction of the Paracetamol and Chloramphenicol at the characteristic
absorption wavelength of 243 and 278 nm.
3.
Results and discussion
Figure 1a shows the surface morphology of ZnO thin
films annealed at 100 oC, with very smooth surface, covered by round
grains. Therefore, these
films have the lowest activity. The
sol-gel films, annealed at 500 oC, are more uniform
and show much
better adhesion of the layers
and the highest density of the film. There is ganglia
typical surface structure of the film. The surface morphology of the samples (Fig. 1b) is
represented by different ganglia-like
hills with typical width of about 1 μm
and a height of 5-10 μm.


(a)
(b)
Fig. 1. SEM images of ZnO thin films annealed at 100oC
(a) and 500oC (b).
XRD patterns of the ZnO thin films are presented in
Fig. 2. The lack of three characteristic peaks of ZnO (Fig. 2a) shows that at
100 oC temperature the material is still in its hydroxide form of
rather amorphous state. The crystallite sizes estimated by the Sherrer,s
formula are about 30.0 nm. Increasing the
annealing temperature causes a transition from orthogonal in to hexagonal
structure, respectively from Zn(OH)2 toward ZnO. The diffraction
peaks of the sol-gel films (annealed at 500 oC, Fig. 2b) can be
indexed to (100), (002), (101), (102), (110), (103), (200), (112) and (201)
diffraction planes at 2θ = 31.77o,
34.42o, 36.25o, 47.54o, 56.60o,
62.86o, 66.37o, 67.96o and 69.09o,
respectively. The films produced are polycrystalline, showing the wurtzite ZnO
hexagonal structure, while there is no evidence for the presence of other
phases.
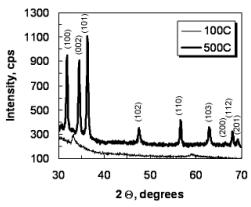
Fig. 2. XRD spectra of ZnO sol-gel films annealed at
100 and 500oC.
Photocatalytic tests are carried out for all the thin
films. Paracetamol and Chloramphenicol are used as a test contaminant. The
initial concentrations of the drugs are 15 and 8 ppm. The pH values
are observed during the photocatalytic reactions (PCA – 5.58
÷ 6.69 and CA – 5.31 ÷ 6.49).
Figure 3
demonstrate the bleaching kinetics of the PCA and CA in aqueous solutions by
ZnO films (annealed at 100, 300 and 500oC) under UV-light illumination.
It can be seen that the degradation efficiency of the thin films increases with
increasing annealing temperature. The experiments
show that the decolorization of PCA and CA under UV illumination follows pseudo
first-order kinetics expressed by ![]() . The slope of logarithmic scale represents linear fits of
the experimental data points by the equation.
. The slope of logarithmic scale represents linear fits of
the experimental data points by the equation.
The films annealed at 300 oC have higher
photocatalytic activity (PCA - k= 0.0081 min-1 and CA - k=
0.1024 min-1) than those, prepared at 100 oC (PCA - k=
0.0053 min-1 and CA - k= 0.0149 min-1). As seen
from the figure, the films obtained at 500 oC have the highest
photocatalytic activity (PCA - k= 0.0362 min-1 and CA - k=
0.1373 min-1). The values of the rate constants are confirmed by rate
of degradation of the pharmaceutical products. The degradation is calculated
using Eq.1.
Nanostructured ZnO films annealed at 500 oC have a highest photocatalytic efficiency (PCA -12.24% and
CA – 39.22% for four hours), while samples obtained at 100 oC
have a lowest activity (PCA -1.99% and CA – 5.88%),
Figure 3.
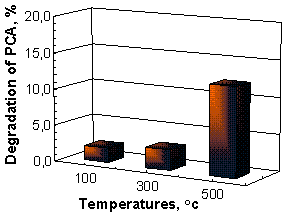
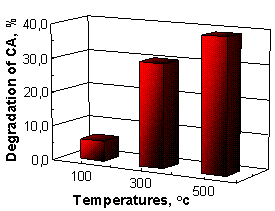
(a) (b)
Fig. 3. Photodegradation curves of Paracetamol (a) and
Chloramphenicol (b) using ZnO thin films as photocatalysts.
4. Conclusion
In summary, ZnO thin films are deposited by sol–gel
method using dip coating technique. The thin films consist of homogeneous and
ganglia-like structures belonging to hexagonal wurtzite structures. The
crystallites sizes are increased with rise of the annealing temperatures. The
enhanced photocatalytic activity of ZnO thin films might be ascribed to the
increase of surface to volume ratio, roughness and mean grain size. The good
performance of ZnO thin films indicates that it can be used as a promising
photocatalyst for the practical application in photocatalytic decolorization of
pharmaceutical products.
Acknowledgements: This research is financially supported by
projects BG051PO001-3.3.06-0050, FP7 project Beyond Everest and
Russian Presidential Program of engineer advanced trading.