Burko D.V.1, Kucharskaya T.A.1, Kvach
S.V.1, Zinchenko À.I.1,2
1Institute of
Microbiology, National Academy of Sciences of Belarus, Minsk
2International Sakharov Environmental University, Minsk
intercalation and release of pharmaceutically useful
diadenosine tetraphosphate from a layered double hydroxide
Layered double
hydroxides (LDHs), also called “anionic clays” are a family of materials that
have attracted increasing attention in recent years due to their technological
importance in catalysis, separation technology, optics, medical science, and
nanocomposite materials engineering. LDHs consist of positively charged metal
hydroxide layers, in which the anions (along with water) are stabilized in
order to compensate the positive layer charges. The general chemical formula of
LDH clays is written as [MII1−xMIIIx(OH)2]x+(An−)x/n·yH2O,
where MII is a divalent metal ion, such as Mg2+, Ca2+,
Zn2+, etc., MIII is a trivalent metal ion, such as Al3+,
Cr3+, Fe3+, Co3+, etc. and An− is an anion of any
type with charge n, such as Cl−, CO32−,
NO3−, etc. [1]. The electrostatic interactions and
hydrogen bonds between layers and contents of the gallery hold the layers
together, forming a three-dimensional structure. There are a number of
combinations of divalent and trivalent cations that can form LDHs. For these
ions, the only requirement is that their radii are not too different from those
of Mg2+ and Al3+. The anions occupy the interlayer region
of these layered crystalline materials. The remarkable behavior of LDHs is
their high reactivity toward various organic anions, which can exchange as much
as 80–100% of the interlayer anions.
LDHs
have historically been of interest as catalysts, ceramic precursors, traps for
anionic pollutans, catalyst supports, ion exchangers, and additives for
polymers. More recently, the successful synthesis of LDH materials on the
nanometer scale has paved the way for many novel applications particularly in
the field of nanomedicine. For example, LDH materials are increasingly explored
as controlled release systems [2–4]. Up to now many kinds of anions were
intercalated in the LDH interlayer gallery, such as common inorganic anions,
organic anions, polymeric anions, complex anions, macrocyclic ligands and their
metal complexes, polyoxalates, and biochemical anions (amino acids, CMP, AMP,
ATP and nucleic acid fragments).
In the
present study, we prepared artificially a novel bio-inorganic nanohybrid of LDH
and biomolecule (pharmaceutically useful diadenosine tetraphosphate [5, 6], Ap4A)
and investigated the possibility of using intercalated LDH as a drug delivery
system.
Materials and methods. Materials. Ap4A
sample was synthesized from ATP enzymatically as previously described in paper
[7]. Twice-distilled water from which carbon dioxide was removed by boiling was
used in all experiments.
Preparation of LDH/Ap4A nanohybrid particles. The LDH/Ap4A
nanohybrid with Mg:Al ratio of 2:1 was synthesized by
coprecipitation and crystallization at 60°C in the presence of ammonium hydroxide.
Briefly, a requisite amount of Mg(NO3)2·6H2O
and Al(NO3)3·9H2O were dissolved in 10 ml of
water. The resultant solution was added dropwise to 10 ml of 10 mM NH4OH
solution containing 10 mM Ap4A at
25°C while stirring vigorously. The pH of mixture was maintained about 10. The
resultant reaction slurry was aged at 55°C for 12 h. The
resulting white precipitate was collected by centrifugation and washed five
times with water, and finally, with acetone. All the samples were air-dried at 60°C
for 5–6 h. To obtain LDH nanoparticles without Ap4A (LDH/NO3)
the above procedure was repeated without addition of Ap4A to
solution of NH4OH.
Characterization of LDH/Ap4A nanohybrids. As-obtained LDH nanoparticles were imaged
using a JEOL JSM-2010 transmission electron microscope at the acceleration
voltage of 200 kV.
The
loading capacity of Mg,Al–LDH/Ap4A nanoparticles and the efficiency
of this process under different experimental conditions were determined by UV spectroscopy. The intercalated amount of
Ap4A in the LDH/Ap4A nanohybrids was determined by 1202 Shimadzu
Corporation model UV-vis spectroscopy using the following method. A known
weight of the nanohybrids was placed in a 10 ml volumetric flask, then 0.5 ml 6
M HCl solution was added, and the balance filled with phosphate buffer solution
(0.02 M). Then the concentration of Ap4A in solution was determined
by monitoring the absorbance at λ=260 nm (ε=30800 M-1·cm-1)
with UV-vis spectroscopy to calculate the intercalated amount of Ap4A
into the nanohybrids.
Release of Ap4A
from LDH/Ap4A nanohybrids. To measure the amount of Ap4A
released from LDH/Ap4A nanohybrids, the in vitro drug release test
was performed at 25ºC by stirring powdered LDH/Ap4A nanohybrids
(0.032 g) in 20 ml either a pH 4.4 or 7.5 0.05 M phosphate-citrate buffer
solution [8]. Aliquots (1 ml) of the suspension were taken at desired time
intervals, centrifuged and the Ap4A content of supernatant was
determined by UV absorbtion at λ=260 nm to calculate the release amount of
Ap4A from the nanohybrids. The percentage released at each time point
was expressed as a fraction of the total amount of Ap4A.
Results and discussion. The LDH, containing
Ap4A (LDH/Ap4A) was prepared by coprecipitation from a
mixed aqueous solution containing Mg2+, Al3+ and Ap4A.
In order to survey the optimal conditions
for intercalation, various reaction conditions, that is, temperature, reaction
time, concentration and pH, were examined. Based on these experiments, it was
concluded that the optimal intercalation conditions of Ap4A are as
follows: Mg:Al molar ratio of 2:1, room temperature, pH 10, and 12 h aging. The maximum amount
of Ap4A in the intercalation compound was around 0.83 mmol per 1 g
of LDH.
The morphology and size of LDH/Ap4A
nanoparticles have been estimated by electron microscopy. Transmission electron
microscopy image of LDH/Ap4A nanoparticles is shown in Fig. 1.

Fig. 1. Transmission
electron microscopy image of LDH/Ap4A nanoparticles
It
should be noted the presence of small hexagonal platelets having a diameter of about 200 nm together with some larger platelets (about 350
nm), likely due to the formation of aggregates.
The
intercalated Ap4A was quantitatively recovered
from the host lattice by treatment of the LDH/Ap4A
nanoparticles with 0.2 M HCl. Therefore it was
concluded that Ap4A is intercalated into LDH without decomposition.
In order
to investigate the possibility of using intercalated LDH as a drug delivery
system, deintercalation of Ap4A was examined. Typical release
kinetic curves of Ap4A from the LDH/Ap4A nanoparticles at different pH are shown in Fig. 2. As can be seen from Fig.
2, the physical mixture LDH/NO3 and Ap4A exposed to buffer solution (pH 7.5) release Ap4A
quickly, the release being complete within 5 min. The release rate of Ap4A
from the nanohybrid is obviously lower than that from the physical mixture. In
addition, the release rate of Ap4A from the nanohybrid is obviously
dependent on pH, and the release rate at pH 7.5 is remarkable lower than that
at pH 4.4.
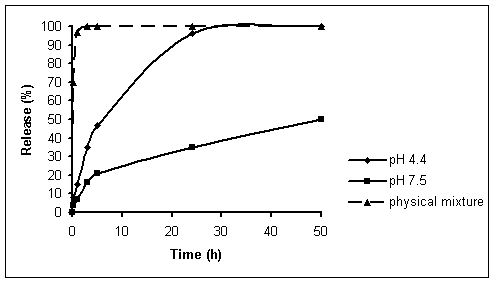
Fig. 2. Release of Ap4A from LDH/Ap4A
nanoparticles
in 0.05 M phosphate-citrate buffer solution at pH 4.4 and pH 7.5
The lower release rate of Ap4A
from LDH/Ap4A nanohybrids at pH 7.5
indicates that the LDH/Ap4A nanohybrids are indeed a potential drug
delivery system. Such a discrepancy of the release rate at pH 4.4 and pH 7.5
may be due to a possible difference in mechanism for the release of Ap4A
from the nanohybrid [9]. At acidic pH, LDHs begin to dissolve. This would
indicate that release of an interlayer molecule should occur mainly through the
removal of inorganic host. At above pH 7, the LDH should be more stable, and as
a result, release may be attributed to the restricted motion of Ap4A molecules arising from steric effect of
LDHs and the electrostatic interaction between Ap4A anions and
positively charged LDHs layers. That is to say, the mechanism of release in the
pH 4.4 environment should be through both the dissolution of LDH layers and the
ion exchange; while for the pH 7.5 release, the mechanism should be primary
through ion exchange with the ions in the buffer solution [9].
In summary, in
this work we for the first time successfully show that Ap4A can be
reversible intercalated into LDH. Therefore, LDH would be attractive candidate
of the studied dinucleotide carrier.
Acknowledgements. The
work was supported by a grant from the Belarus State Research Program
«Fundamental Basics of Biotechnologies».
References
1. Cavani F., Trifiro F.,
Vaccari A. Hydrotalcite-type anionic clays: preparation, properties and applications
// Catal. Today. 1991. Vol. 11. P. 173–301.
2. Choy J.H., Park M.
Nanohybridization of biomolecules with layered double hydroxides and hydroxy
double salts for advanced applications // Clay Sci. 2005. vol. 12 (Suppl. 1).
P. 52–56.
3. Xu
Z.P., Lu G.Q. Layered double hydroxide
nanomaterials as potential cellular drug delivery agents // Pure Appl. Chem.
2006. Vol. 78. P. 1771–1779.
4. Hussein
M.Z., Al Ali S.H., Zainal Z., Hakim M.N. Development of antiproliferative
nanohybrid compound with controlled release property using ellagic acid as the
active agent // Int. J. Nanomed. 2011. Vol. 6. P. 1373–1383.
5. Kikuta Y., Ohiwa E., Okada K.,
Watanabe A., Haruki S. Clinical application of diadenosine tetraphosphate
(Ap4A:F-1500) for controlled hypotension // Acta Anaesthesiol. Scand. 1999. Vol. 43. P. 82–86.
6. Guzman-Aranguez A., Loma P., Pintor J. Focus on molecules: diadenosine tetraphosphate //
Exp. Eye Res. 2011.
Vol. 92. P. 96–97.
7. Burko
D.V., Eroshevskaya L.A., Kvach S.V., Shakhbazau A.V., Kartel N.A.,
Zinchenko A.I. Application of recombinant enzymes for the synthesis of
pharmaceutically valuable nucleosides and nucleotides // Biotechnology in Medicine, Foodstuffs, Biocatalysis,
Environment and Biogeotechnology / Eds: S.D. Varfolomeev, G.E. Zaikov, L.P. Krylova. New
York, Nova Science Publishers, Inc. 2010. Ð. 1–13.
8. Liu C., Hou W., Li
L., Li Y., Liu S. Synthesis and characterization
of 5-fluorocytosine intercalated Zn–Al layered double hydroxide // J. Solid State Chem. 2008. Vol. 181. P. 1792–1797.
9. Tyner
K.M., Schiffman S.R., Giannelis E.P. Nanobiohybrids
as delivery vehicles for camptothecin // J. Control Rel. 2004.
Vol. 95. P. 501–514.