Lozhnikova N.V.2, Beresnev A.I.1,
Kucharskaya T.A.1,
Kvach S.V.1,
Eroshevskaya L.A.1,
ZinchenkoÀ.I.1,2
1Institute of
Microbiology, National Academy of Sciences of Belarus, Minsk,
2International Sakharov Environmental University, Minsk
biocatalitic synthesis of
5-fluoro-2′-deoxyuridine by thymidine phosphorylase
Non-natural nucleosides are of interest as antiviral and antitumor
agents ([for a review, see [1]). In most cases these molecules have been
synthesized by a stereoselective coupling reaction of the base analogue and the
protected pentose. Bioconversion reactions catalyzed by the action of microbial
enzymes (including nucleoside phosphorylases), as reported in literature, make
an alternative approach for preparing nucleosides, nucleotides and their
modified analogues instead of conventional chemical syntheses, plagued by
formation of regio- and stereochemical isomers and by low overall yields [2].
Thymidine phosphorylase of Escherichia
coli (EC
2.4.2.4; TP) catalyzes conversion of thymidine to thymine and
2-deoxy-D-ribose-1α-phosphate [3–5]. TP can also perform the
transglycosylation of thymidine and its analogues, including
2′-deoxyuridine (2'-dUrd), because the phosphorolysis reaction is
reversible. The synthesis of modified nucleosides using bacterial TP is well
documented, including our papers [4, 6, 7] but in most cases it was conducted
with non-recombinant enzymes.
5-Fluoro-2'-deoxyuridine (5-FdUrd), a derivative of
5-fluorouracil (5-FUra), is an anti-metabolite showing a
significant cytotoxic activity [8].
This drug is widely used for the treatment of some solid tumors [9, 10]. Chemical
synthesis is the primary method for 5-FdUrd production. It usually involves
environmentally unfriendly procedures and has low enantioselectivity, thus
reducing process efficiency
and increasing downstream costs.
The present study was undertaken in order to develop a practical
synthesis of 5-FdUrd using reaction of enzymatic transglycosylation.
Materials and methods. 2'-dUrd
was chosen as a donor of the ß-D-deoxyribofuranose moiety and 5-FUra as
its acceptor. Recombinant TP isolated
from cells of the newly engineered strain E.
coli TDP served as biocatalyst [11]. Optimal procedure for the reaction:
2'-dUrd (0.1
mmol, 22.8 mg), 5-FUra (0.1
mmol, 13 mg), and TP (15 units) from E.
coli were added to 10 mL of 2 mM phosphate buffer (pH 7.0). The reaction mixture was stirred at
40ºC for 1 h. The reaction was monitored by thin-layer chromatography on
Merck F254 silica gel 60 aluminium sheets as described in [11].
Results and discussion. First, we seeked for the
most suitable reaction conditions for each concentration of the substrates (2'-dUrd, 5-FUra, and phosphate ion). The studied reaction catalyzed
by TP proceeds in two steps (Fig. 1, step 1 and step 2) and both are
reversible. Therefore, the produced uracil and the concentration of phosphate
buffer inhibit step 2 under «one-pot»
reaction conditions.
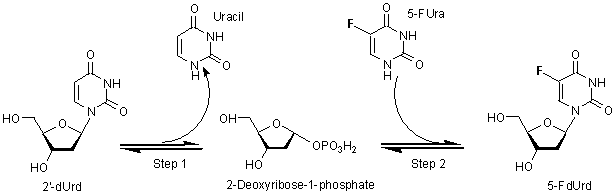
Fig.
1. Synthesis of 5-FdUrd from 2'-dUrd and 5-FUra by recombinant TP
Fig. 2 shows the effects of
substrate concentrations (2'-dUrd and 5-FUra). As can be seen from Fig. 2a, with
increase in 5-FUra concentration, the initial velocity decreased, and the
conversion level increased. But 5-FUra at 100 mM concentration shifted the
equilibrium and inhibited the production of 5-FdUrd.
Fig. 2b shows the effect of
the 2'-dUrd concentration. The conversion and initial velocity of 5-FdUrd
production increased with increasing concentration of 2'-dUrd. However,
starting from 5:1 ratio of 2'-dUrd to 5-FUra the reaction yield reached the
quantitative level.
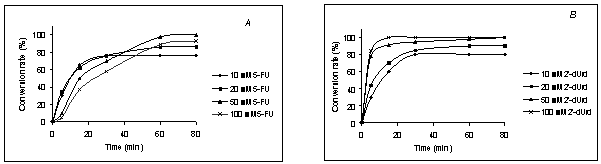
Fig. 2. Effects of variation of
substrate concentration on the conversion rate.
(A) Effect
of changes in 5-FUra concentration (2'-dUrd
concentration was kept constant at 10 mM). (B) Effect of changes in 2'-dUrd
concentration (5-FUra concentration was kept
constant at 10 mM).
The increasing concentration of phosphate ions inhibited the catalytic
conversion of 2'-dUrd to 5-FdUrd (not shown). We suggest to use the 5:1 ratio
of 5-FUra (50 mM) to 2'-dUrd (10 mM) and 2 mM phosphate buffer (pH
7.0).
In conclusion, we report in this communication the first application of
recombinant E. coli TP to produce
5-FdUrd from 2'-dUrd and 5-FUra. The obtained results open up possibilities for
engineering of industrial technology for production of the title
pharmaceutically valuable modified nucleoside.
References:
1. Mikhailopulo I.A., Miroshnikov A.I. New
trends in nucleoside biotechnology // Acta Natur. 2010. Vol. 2, ¹ 2. P. 36−58.
2. Vorbruggen H., Ruh-pohlenz C. Handbook of Nucleoside Synthesis. John
Wiley & Sons, Inc., New York etc, 2001. 631 pp.
3. Walter M.R., Cook W.J., Cole L.B., Koszalka G.W.,
Krenitsky T.A., Ealick S.E. Three-dimensional
structure of thymidine phosphorylase from Escherichia coli at 2.8 A resolution // J. Biol. Chem. 1990. Vol. 265. P.
14016−14022.
4. Utagawa T. Enzymatic preparation of
nucleoside antibiotics // J. Mol. Catal. B: Enzym. 1999.
Vol. 6, N 3, P. 215−222.
5. Panova N.G.,
Alexeev C.S., Polyakov K.M., Gavryushov S.A., Kritzyn A.M., Mikhailov S.N. Substrate specificity of thymidine phosphorylase of E. coli: role of hydroxyl groups // Nucleosides Nucleotides Nucleic Acids. 2008.
Vol. 27, N 12. P. 1211–1214.
6. Kalinichenko E.N., Barai V.N., Bokut S.B.,
Zinchenko A.I., Herrmann G., Mikhailopulo I.À. Microbiological synthesis of 5-ethyl- and (E)-5-(2-bromovinyl)-2'-deoxyuridine
// Biotechnol. Lett. 1989. Vol. 11, N 9. Ð. 621−626.
7.
Serra I., Ubiali D., Albertini A.M., Amati G., Daly S., Terreni M. Microbial
nucleoside phosphorylases as efficient biocatalysts for the synthesis of
antiviral and antitumoral nucleosides // J. Biotechnol. 2010. Vol. 150S. P.
408.
8. Uchikubo Y., Hasegawa T., Mitani S., Kim H.S., Wataya Y.
Mechanisms of cell death induced by 5-fluoro-2'-deoxyuridine (FUdR) – Necrosis or apoptosis after treated with FUdR
// Nucl. Acids
Symp. Ser. 2002. 2, N 1. P. 245–246.
9. Chu S.I, Kim
H.J. Skubitz K. Pulmonary
toxicity of continuous infusion 5-fluoro-2'-deoxyuridine // Anti-Cancer
Drugs. 1995. Vol. 6, N 3. P. 475–478.
10. Weinreich J., Schott S., Konigsrainer I., Zieker D.,
Konigsrainer A., Schott H. Cytostatic activity of the duplex drug linking
2′-deoxy-5-fluorouridine (5FdU) with 3′-C-ethynylcytidine (ECyd)
against gastric adenocarcinoma cell lines // Invest. New Drugs. 2011. Vol. 29.
P. 1294–1302.
11. Burko D.V.,
Eroshevskaya L.A., Kvach S.V., Shakhbazau A.V., Kartel N.A., Zinchenko
A.I. Application of recombinant enzymes for the synthesis of pharmaceutically
valuable nucleosides and nucleotides Biotechnology in Medicine, Foodstuffs, Biocatalysis,
Environment and Biogeotechnology / Eds: S.D. Varfolomeev, G.E. Zaikov, L.P. Krylova. – New
York, Nova Science Publishers, Inc. – 2010. Ð. 1–13.