Andriychuk
N.D., Kalyuzhnyi G.S., Kovalenko A.A., Lyshtvan E.Y.
East
Ukrainian National University named after Vladimir Dali
CONVECTION EFFECT ON HEAT EXCHANGE IN OPTOPNEUMATIC CELL
INTRODUCTION
Optopneumatics is
one of the modern directions in automatics. Its basic element is an
optopneumatic cell (ÎPC) [1]. This cell
represents a hermetic gas-filled cylindrical container (fig. 1). The modulated
luminous energy from a laser enters the inner gas-filled cavity 2 of the cell
through transparent cover 1 (in our experiments the cover was made of acrylic resin).
The cavity is located in the control channel of a fluidic amplifier. The
luminous energy falls onto absorption layer 3 of black carbon deposited on the
back wall of the cell. This layer absorbs the radiant energy converting it into
the thermal one. Its temperature increases and, due to thermal diffusion, so
does the temperature of the gas layers adjoining the absorption layer and the
whole gas in the cell. The increase of the temperature in the confined space
results in the rise of the pressure. In the case of pulse input of the radiant
energy, there arises a pressure pulse in the optopneumatic cell.
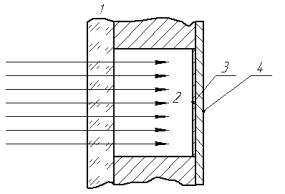
Fig. 1.
Optopneumatic cell
The pressure pulse
propagates in the control channel through membrane 4 to the power jet resulting
in its deviation.
The temperature field in the cell changes due to the action of several
mechanisms – thermal radiation, thermal conduction, and convection. Under the
real conditions, all the three mechanisms are realized simultaneously, and the
process is called a complex energy exchange. However, the contribution of
separate mechanisms of energy exchanges into the common process is different,
and in individual cases some of them can be neglected. Our previous
investigations [2] demonstrated that, considering heat exchange in an OPC
filled with diatomic gas, one can omit the radiant energy, as its contribution
into the energy exchange is negligibly small.
The purpose of the present paper lies in the determination of the role
of convection in the process of heat exchange in an ÎPC.
MATHEMATICAL MODEL OF HEAT EXCHANGE PROCESSES IN OPC
Studying complex phenomena depending on several time- and space-varying
physical parameters, their interrelation can be expressed by a system of
differential equations. Most often, especially in the case of nonlinear
equations, such a system must be solved numerically.
The set of
equations for thermogravitational convection of incompressible liquid in the
Boussinesq approximation includes: the Navier-Stokes equation with regard for
the buoyancy force, the heat conduction equation, and the continuity equation
under the condition of incompressibility of the gas [3]. The physical meaning
of the Boussinesq approximation consists in neglecting density variations caused by the
change of the temperature everywhere, except for the expression describing the
buoyancy force.
![]() ;
;
![]() (1)
(1)
![]()
Here, ![]() denotes the gas density,
denotes the gas density, ![]() is the velocity,
is the velocity, ![]() is the pressure,
is the pressure, ![]() stands for the dynamic
viscosity,
stands for the dynamic
viscosity, ![]() is the temperature coefficient of volumetric expansion,
is the temperature coefficient of volumetric expansion, ![]() is the temperature, and
is the temperature, and ![]() is the free fall acceleration.
The temperature and pressure are reckoned from the initial values.
is the free fall acceleration.
The temperature and pressure are reckoned from the initial values.
The system must be
supplemented with initial and boundary conditions. They are presented by the
initial gas temperature, the increase of the pressure induced by the former,
the temperature of the OPC walls, and the requirement of gas adhesion at the
walls.
In the cylindrical
coordinate system![]() , the axis
, the axis ![]() coincides with the OPC axis,
while the origin is located in the middle of the axis. In view of the axial
symmetry of the problem, it is sufficient to solve it for the two-dimensional
region formed by one half of the cylinder cross section
coincides with the OPC axis,
while the origin is located in the middle of the axis. In view of the axial
symmetry of the problem, it is sufficient to solve it for the two-dimensional
region formed by one half of the cylinder cross section ![]() . Moreover, one must specify at the axis the conditions of the absence
of a thermal flow for the temperature and slip for gas. In this case, the
boundary conditions will have a form
. Moreover, one must specify at the axis the conditions of the absence
of a thermal flow for the temperature and slip for gas. In this case, the
boundary conditions will have a form
![]() ;
;
![]() (2)
(2)
![]() .
.
(where ![]() and
and ![]() are OPC radius and height,
correspondingly), and the initial conditions are presented as
are OPC radius and height,
correspondingly), and the initial conditions are presented as
![]() (3)
(3)
![]() .
.
Here, ![]() is the initial temperature,
is the initial temperature, ![]() is the universal gas
constant and,
is the universal gas
constant and, ![]() is the molar mass of the gas.
is the molar mass of the gas.
NUMERICAL SOLUTION
OF THE MODEL EQUATIONS
As a numerical method, we chose the control volume approach that
represents one of the modifications of the conservative methods. Its basic idea
can be directly interpreted. The calculation region is divided into some number
of nonoverlapping control volumes in such a way that each node is located in
one control volume. The differential equation for the sought quantity ![]() is integrated over each control volume. The integrals are
evaluated using piece profiles that describe the variation of
is integrated over each control volume. The integrals are
evaluated using piece profiles that describe the variation of ![]() between nodes. As a
result, one obtains a discrete analogue of the differential equation that
includes the values of
between nodes. As a
result, one obtains a discrete analogue of the differential equation that
includes the values of ![]() in several nodes.
in several nodes.
The discrete analogue obtained in such a way expresses the law of
conservation of ![]() for a finite control volume the same way as the
differential equation expresses the conservation law for an infinitesimal
control volume.
for a finite control volume the same way as the
differential equation expresses the conservation law for an infinitesimal
control volume.
Thus, the control volume approach ensures the exact integral
conservation of such quantities as mass, momentum, and energy for any group of
control volumes and, consequently, for the whole calculation region. This
property is valid at any number of nodes, and not only in the limiting case of
their very large number. Thus, even the solution at a rough mesh satisfies
accurate integral balances.
INVESTIGATION RESULTS
The mathematical
model allowed us to obtain rather complete information on the thermal processes
taking place inside the ÎPC. These data
allow one to obtain qualitative information on the character of gas motion and
temperature distribution.
The question of
practical interest is the effect of convection on the heat exchange process in
an OPC shown in Fig. 2. One can see that, at the beginning of the process of
cell cooling, the effect of convection on the general process is negligibly
small and heat exchange is determined only by the thermal conduction mechanism.
Such a situation conserves until the gas temperature in the cell decreases
approximately twofold as compared to the initial value. It can be explained by
the fact that the establishment of convective gas motion requires a finite
time, i.e. the convection process is inertial. It is worth noting that the
obtained data are in good agreement with experiment [4].
After that, the
steady-stated convective gas motion results in a considerable increase of the
cooling rate. Due to this fact, the total time of gas cooling in the cell decreases
approximately twice.
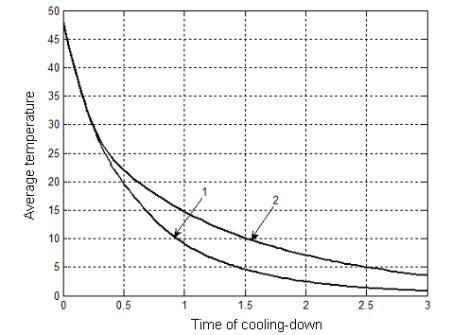
Fig. 2. Variation
of the mean gas temperature in the cell (a cell 30 mm in diameter and 60 mm in
length with regard for convection (1) and thermal conduction (2).
SUMMARY
1. The
mathematical model of heat exchange in an ÎPC with regard for
mechanisms of thermal conduction and convection is considered.
2. The
performed calculations have shown that the contribution of convection at the
beginning of the process of gas cooling in the cell is inessential, but it increases
with time. Due to this fact, the total time of gas cooling in the cell can
decrease approximately twice.
3. The
obtained results will allow one to improve methods of computation of time
characteristics of ÎPCs in automatic
control systems.
REFERENCES
1.
Êîâàëåíêî À.À., Ëûøòâàí
Å.Þ., Ñîðîêà Ñ.È. Îïòîïíåâìàòèêà â òåõíèêå: ìîíîãðàôèÿ. – Ëóãàíñê: èçä-âî ÂÍÓ
èì. Â Äàëÿ,2008. – 184 ñ.
2.
Ëûøòâàí Å.Þ. Äèíàìèêà
ëó÷èñòîãî òåïëîîáìåíà â îïòîïíåâìàòè÷åñêèõ óñòðîéñòâàõ / Åëåíà Þðüåâíà Ëûøòâàí – Ëóãàíñê: ³ñíèê ÑÍÓ ³ì. Â. Äàëÿ, 2007. - ¹4 (110). – ñ. 149-155.
3.
Ãèäðîäèíàìèêà/ Ëàíäàó Ë.Ä., Ëèâøèö Å.Ì. -
Ìîñêâà: Íàóêà, 1988 – 733ñ
4. L.C. Aamodt, J. C.
Murphy, J.G. Parker. Size Considerations in the Design of Cells for
Photoacoustic Spectroscopy/ Journal of Applied Physics, Vol. 48,- 3, March 1977, p.p. 927-933.