*120161*
O. Mykoliv1, Î. Kovalchuk1, R. Vynnytska1, L. Zhurakhivska1, N. Marintsova1, N.Ruda2, N.Chornoivan2,
G. Stepanuyk2, A. Komar3,
G. Zagoriy3, M. Ponomarenko4, V. Novikov1
1Department of Technology of
Biologically Active Substances, Pharmacy and Biotechnology, National University
“Lviv Polytechnic”, 79013, Ukraine, Lviv-13, Bandera Str. 12, e-mail: vnovikov@polynet.lviv.ua
2Vinnytsia National N.I. Pyrogov Memorial Medical University,
21018, Ukraine, Vinnytsia, Pyrogov Str, 56
3PJSC “Pharmaceutical company”
Darnitsa”, 02093, Ukraine, Kyiv, Boryspilska Str., 13
4 P.L.Shupik National Medical Academy
of Post-Graduate Education, 04112, Ukraine, Kyiv, Dorogozhytska Str., 9
Synthesis of new
amino acid derivatives of 1,4-naphthoquinone and research of their biological
activity
One of the chapters of up-to-date
pharmaceutical and organic chemistry is the chemistry of quinoid compounds,
where an important place is occupied by a naphthoquinone and its derivatives. Compounds of these class are interesting due
to its physiological, chemical and physico-chemical properties, that
predetermine a variety of high biological activity of 1,4-naphthoquinone
derivatives.
As it is known, amino acids are
widely used in medical practice. It is explained by their ability to
participate in a nitrous exchange, in the synthesis of necessary for normal
viability of organism proteins, enzymes, hormones, etc. Therefore with the aim
of obtaining new potential drugs, undeniable interest is presented by
researches in the synthesis of compounds that contain amino acid fragments and
quinoid bond system simultaneously.
For identifing of new
physiologically active compounds in the series of quinones the aminoacid
derivatives of 1,4-naphthoquinone were synthesized and acute toxicity, anti-ischemic and antihypoxic activities were
investigated.
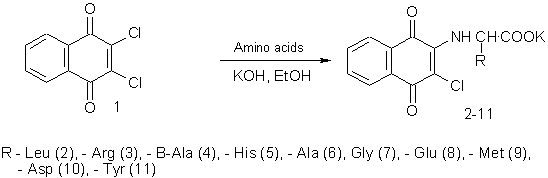
With the aim of synthesis of amino
acid derivatives of 1,4-naphtoquinone as initial compound
2,3-dichloro-1,4-naphthoquinone (1) was used. It is able to exchange enough
movable atoms of chlorine for various nucleophilic reagents. As amino acid
components, α- and
β- alanine,
histidine, arginine, leucine, methionine, tyrosine, aspartic and glutamic acids
were used.
Endurance of
2,3-dichloro-1,4-naphthoquinone (9) with the equimolar amount of corresponding
amino acid in a hydroalcoholic medium at 60 oÑ during 6 hours in presence of KOH
or potassium carbonate allowed to get with good yields of corresponding amino
acid substituted 1,4-naphthoquinones. For providing better watersolubility to
obtained compounds, with the aim of further biological screening, potassium
salts were synthesized (2-11).
The structure of obtained compounds was confirmed by the results
of element analysis and spectral data. In IR-spectras of amino acid derivatives
of 1,4-naphthoquinone characteristic bands of absorption of valency vibrations
of quinoid carbonyl groups and –COOH fragment at 1664-1724 cm-1, in
area of ~3400 ñm-1 - bands
of valency vibrations of NH- groups
that interflow with extended bands of absorption of hydroxylic groups. In 1H
NMR-spectras the proton signals are corresponded to the integral intensities
and offered structure.
The acute toxicity of the
synthesized substances was studied on white nonlinear mise, antihypoxic,
anti-ischemic, anticonvulsant, actoprotectant
properties and influence on cerebral circulation of blood - on nonlinear
rats.
Characterizing data of acute
toxicity it is possible to mark that derivatives 2,4,5,6 and 9 according to
classification of Sydorova K.K. on the dimension of index LD50 can
be attributed to the low-toxic substances, as their LD50 is within
the limits of 225-400 mg/kg. Compounds 3,7, 8, 10 and 11 can be attributed to
the very low toxic substances, as their index of LD50 is more than
500 mg/kg and is within the limits of 540-650 ìã/êã.
Undertaken studies showed that
potassium salts of amino acid derivatives of 1,4-naphthoquinone have
antihypoxic, anti-ischemic, cerebroprotectant activities and have stimulant
action on the blood supply of cerebrum. Some of the compounds of these set(5,6,7,8,10) according to the value of
anti-ischemic effect on the models of acute violation of cerebral supply in
doses that are 1-10% from LD50, is competitive with cavinton
(5mg/kg) and with piracetam (100 mg/kg), and for a level of the antihypoxic
action don’t yield to standard preparation of emoxipin (10 mg/kg). For compound
6 expressive stimulant action on cerebral circulation of blood is
characteristic, in a magnitude and duration it twice exceeds the effect of
cavinton. For the indicated compound anticonvulsant action is inherent, but it
is the weaker than the standard cerebroprotectant carbamazepine.
The wide spectrum of pharmacological
properties of the synthesized substances caused the research of actoprotectant compounds among them. Most
efficiency was shown by compounds 6,7, that in the level of activity prevailed
the standard preparation of bemithyl.
For compounds-leaders
actoprotectant action in the terms of hypo- and hyperthermia was studied
and the possible mechanism of their action was studied out. Results of
undertaken studies showed that course of medication (14 days) to the organism
of rats of compound 7 assists to adaptive alteration of metabolic processes due
to strengthening of aerobic reactions, activation of lipolysis (as an
additional source of energy) and antioxidant defence that assists to the
improvement of energy supply of skeletal muscles and increase of physical
endurance.