Chemistry and Chemical Technologies / 4. Chemical and
Pharmaceutical Industry
Dr. Valery V. Belakhov1, Dr. Sc., Prof.
Aleksandr V. Garabadzhiu2a,
Dr. Sc., Prof. Boris I. Ionin2b
1Schulich Faculty of Chemistry, Technion – Israel Institute
of Technology Israel; 2aDepartment of Technology of Microbiological
Synthesis and 2bDepartment
of Organic Chemistry, Saint-Petersburg State Technological Institute
(Technical
University), Saint-Petersburg, Russia
chvalery@techunix.technion.ac.il
DIALKYLAMIDOPHOSPHATE
DERIVATIVES OF AMPHOTERICIN B: PREPARATION AND ANTIFUNGAL ACTIVITY
1.
Introduction
Amphotericin B is the main polyene macrolide antifungal antibiotic due to its high activity and broad spectrum of activity [1, 2]. Recently the range of its medical application has expanded in connection with its use in combination with synthetic antifungal drugs for therapy of fungal infections [3-5] and also the discovery of its antiviral [6, 7] and antitumor activity [8]. Furthermore, amphotericin B is promising in the treatment of leishmaniasis [9, 10] and is used as an anti-inflammatory drug [11], in gene therapy [12], and in combined therapy of serious fungal sepsis together with antibodies [13, 14]. Amphotericin B is especially important for treating fungal diseases that complicate AIDS [15-17]. Nevertheless, the high toxicity (in particular, nephrotoxicity) of amphotericin B [18, 19], the low absorption from the gastrointestinal tract and poor penetration into cerebrospinal fluid [3-5], a whole series of side reactions [20], and the reduced sensitivity to it of pathogenic fungal microorganisms [21, 22] have stimulated vigorous searches for different derivatives of this polyene antibiotic. The preparation of semisynthetic derivatives of amphotericin B with improved chemotherapeutic properties has been reviewed several times [17, 23-25]. It has also been demonstrated that liposomal amphotericin B is one of the modern highly effective drugs of this antibiotic with reduced toxicity [26-28]. We have previously prepared hydrophosphoryl [29], fluoroorganic [30] and N-benzyl derivatives of amphotericin B [31].
2. Experimental part
2.1. Experimental chemical
part
We used commercial amphotericin B (Sigma, USA) with biological
activity 740 ED/mg with specific absorption indices (E1%1cm)
782, 1373, and 1564 at wavelengths 362, 383, and 405 nm, respectively. Organic
solvents were purified by the literature methods [32]. The 1H and 13C
NMR spectra were obtained on a Bruker Avance instrument (Germany) at 500 MHz
(for protons) for 10-15% solutions in DMSO-d6, internal
reference TMS. The 31P NMR spectra were registered on a Bruker
AC-200 instrument (Germany) with operating frequency 80 MHz (200 MHz for
protons), external reference 85% H3PO4. The IR spectra
were registered on a Bruker Vector 22 spectrophotometer (Germany) from KBr
pellets. The UV spectra were recorded on an Ultrospec 2100 Pro-instrument
(Biochrom, UK). The purity of the synthesized compounds was monitored using TLC
on Silica Gel 60 F254 plates (0.25 mm, Merck, Germany) and two
solvent systems CHCl3:MeOH:propan-1-ol:borate buffer (pH 3.14,
3:2:2:1, 1) and EtOH:NH4OH (25% aqueous):H2O (8:1:1, 2).
Compounds were developed using UV light. The sorbent was Silica Gel 60 (63 –
200 mm, Merck, Germany).
2.2. Experimental
biological part
Determination of antifungal activity of hydrophosphoryl
derivatives of amphotericin B II and III relative to six test-cultures of yeast-like
fungi of the genus Candida was performed by serial
dilutions in liquid nutrient medium. The method is based on a serial two-fold
dilution of the compounds under study. The minimum fungistatic concentration
(MFK) was found by the results of visual evaluation of growth rate of a test
culture in the experimental and control tubes on the basis of three
replications. Amphotericin B was used as reference in all biomedical tests.
3. Results and discussion
In continuation of research on the preparation of
semisynthetic derivatives of
amphotericin B (I) with improved medical and biological properties, we studied
the reactions of this heptaene macrolide
antibiotic with dialkylphosphites in the presence of organic base and
produced the corresponding dialkylamidophosphate
derivatives II and III:
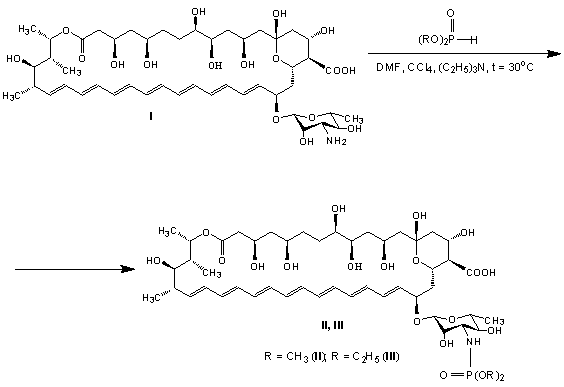
The reaction can be considered as a variation of Todd-Atherton reaction [33, 34], whose synthetic opportunities were generalized in [35].
The resulting derivatives II
and III were solids without distinct melting points that decomposed
on heating. Compounds II and III were very soluble in DMSO and
DMF; slightly soluble in MeOH, EtOH, pyridine, and dioxane; and insoluble in water, acetone, CHCl3, ether,
benzene, and hexane.
The structure of the derivatives is confirmed by 1H, 13C,
and 31P NMR, IR and UV spectroscopy. In the 1H
NMR spectra of compounds II and III contained resonances for protons typical of
amphotericin B [36, 37], protons of methoxy groups performed by doublet signals
(δ 3.32–3.40 ppm) and protons of ethoxy groups consist of triplet signals of methyl groups (δ 1.12–1.25
ppm) and quartet signals of methylene groups (δ 3.81–3.94 ppm). The
constant spin-spin interaction from the phosphorus atom [JHÐ(CH2O-P)] was determined as 10,0-10,2 Hz. The proton at the nitrogen atom (N-H)
observed as a broad singlet (δ 5.12–5.23 ppm). In 13C NMR
spectra there are signals of carbon atoms of amphotericin B [36, 37], and doublet signals of ethoxy groups were
recorded (δ 16.3–17.4 ppm) with the constant of spin-spin interaction JCP 6.7-7.0 Hz. The group OCH2
resonances as doublet in more weak field (δ 61.5-62.7 ppm) with the constant spin-spin
interaction JCP
5.3-5.7 Hz. These were consistent with those
reported in the literature [38]. 31P Chemical shifts δ
of the synthesized derivatives of amphotericin B II
and III are
15.42–15.84 ppm, that is typical for dialkylamidophosphates with tetra-coordinate phosphorus atom [39].
In IR spectra of compounds II and III in addition to absorption
bands belonging to original amphotericin B [40] there are those in regions of
1230–1234 cm–1 and 3957–3962 cm–1 that proves the
presence of phosphoryl group P=O and N–H bonds, respectively. The electronic absorption spectra of II and III
had maxima at 362, 383, and 405 nm,
which indicated that a heptaene conjugated
system was present.
Biological investigations showed that dialkylamidophosphate derivatives of
amphotericin B II and III had high antifungal
activity against six test cultures of the genus Candida: Candida
albicans, Candida utilis, Candida tropicalis, Candida
krusei, Candida parapsilosis and Candida guillermondii, and
minimal fungistatic concentration varied form 0.07 to 6.15 mkg/ml-1.
4. Conclusion
Thus, further search for semi-synthetic derivatives of
amphotericin B under
the Todd-Atherton
reaction conditions is a promising
direction for synthesis of new potent antimycotic preparations with high
antifungal activity against fungal pathogens.
5. Acknowledgments
This work was supported by the Ministry of Education and Science of
Russian Federation under the framework of Federal Program "Scientific and
Scientific Educational Specialists of the Innovational Russia" during
2009-2013 (grant No. 2012-1.5-12-000-1013-005).
6. Literature
1. Gallis H.
A., Drew R. H., Pickard W. W. - Rev. Infect. Dis., vol. 12, No. 2, 308-329,
1990.
2. Lemke A., Kiderlen A.F., Kayser
O. - Appl. Microbiol. Biotechnol., vol. 68. No. 2, 151-162, 2005.
3. Padeiskaya
E. N., Baklanova, O. V. - Pharm. Chem. J., vol. 27, No. 4, 12-23, 1993.
4. Polak A. -
Mycoses, vol. 42, No. 5-6, 355-370, 1999.
5. Antifungal Agents: Advances and Problems
(Ed. Jucker E.), Basel: Birkhauser Verlag. 2003. 248 P.
6. Shneider
M.A. - Mol. Genet. - Mikrobiol. Virusol., No. 5, 41- 46, 1984.
7. Shneider
M.A., Chizhov N.P. - Vopr. Virusol., vol. 31, No. 1, 18-31, 1986.
8. Feigin
A.M. - Med. Hypotheses, vol. 52, No. 5, 383-388, 1999.
9. Ouellette
M., Drummelsmith J., Papadopoulou B. - Drug Resist. Updates, vol. 7, No.
4-5, 257-266, 2004.
10. Kafetzis
D.A., Velissarion I.M., Stabouli S., et al. - Int. J. Antimicrob. Agents, vol.
25, vol. 1, 26-30, 2005.
11. A.Hartl,
E.Leistner, C.Pullen, et al. - Pharmazie, vol. 57, No. 3, 218-223, 2002.
12.
C.Garcia-Chaumont, O.Seksek, J.Grzybowska, et al. - Pharmacol. Ther., vol. 87,
No. 2-3, 255-277, 2000.
13. Matthews
R.C., Burnie J.P. - Vaccine, vol. 22,
No. 7, 865 – 871, 2004.
14. Matthews
R.C., Burnie J.P. - Curr. Mol. Med., vol. 5, No. 4, 403 – 411, 2005.
15. Marques
S.A., Robles A.M., Tortorano A.M., et al. - Med. Mycol., vol. 38, No. 1,
269-279, 2000.
16. Marty F.,
Mylonakis E. - Expert Opin. Pharmacother., vol. 3, No. 2, 91-102, 2002.
17. Zotchev
S.B. - Curr. Med. Chem., vol. 10, No.
3, 211 – 223, 2003.
18. Fanos V.,
Cataldi L. - J. Chemother. (Firenze), vol. 12, No. 6, 463-470, 2000.
19. Deray G.
- J. Antimicrob. Chemother., vol. 49, Suppl. S1, 37-41, 2002.
20. Gallis
H.A., Drow R.H., Pickard W.W., - Rev. Infect. Dis., vol. 12, No. 4,
308-329, 1990.
21. Vanden
Bossche H., Dromer F., Imprivisi L., et al. - Med. Mycol., vol. 36, Suppl.
1, 119-128, 1998.
22. Stevens
D.A., Holmberg K. - Curr. Opin. Anti-Infect. Invest. Drugs, vol. 1, No.
3, 306-317, 1999.
23. Shenin Yu.D., Belakhov
V.V., Araviiskii R.A. - Pharm. Chem. J., vol. 27, No. 2, 14-21, 1993.
24. Shenin
Yu.D., Belakhov V.V. - Antibiot.
Khimioter., vol. 42, No.4, 34-46, 1997.
25. Solov'eva S.Å.,
Olsuf'eva Å.N., Preobrazhenskaya Ì.N. - Usp. Khim., vol. 80, No. 2, 115-138, 2011.
26. Herbrecht
R., Natarajan-Ame S., Nivoix Y., Letscher-Bru V. - Expert Opin. Pharmacother.,
vol. 4, No. 8, 1277 – 1287, 2003.
27. Martino
R.. - Curr. Med. Res. Opin., vol. 20, No. 4, 485-504, 2004.
28. Gibbs W.
J., Drew R.H., J. R. Perfect J.R., Exp. Rev. Anti-Infect. Ther., vol. 3, No.
2, 167-181, 2005.
29. Belakhov
V.V., Shenin Yu.D., Araviiskii R.A., Sthil'bans E.B. - Antibiot. Khimioter.,
vol. 41, No. 7/8), 4-8, 1996.
30. Shenin
Yu.D., Belakhov V.V., Shatik L.I., Araviiskii R.A., Antibiot. Khimioter., vol.
43, No. 12, 8-11, 1998.
31. Belakhov
V.V., Shenin Yu.D. - Pharm. Chem. J., vol. 41, No. 7, 362-366, 2007.
32. Armarego, W.L.F. and
Chai, C.L.L., Purification of Laboratory Chemicals, Oxford:
Butterworth-Heinemann Press, 1024 p., 2012.
33. Atherton F.R., Openshaw
H.T., Todd A.R. - J. Am. Chem. Soc., No. 2, 382-385, 1945.
34. Atherton F.R., Howard
H.T., Todd A.R. - J. Am. Chem. Soc., No. 9, 1106-1011, 1948.
35. Nifant'ev E.E.
Chemistry of hydrophosphoryl compounds [in Russian],
M.: Nauka, pp. 120-131, 1983.
36. Brown J.M., Sidebottom P.J. - Tetrahedron, vol. 37, No. 7, 1421-1428,
1981.
37. Sowinski P., Pawlak J., Borowski E. -
Magnet. Resonance in Chem., vol. 30, No. 4, 275-279, 1992.
38. Panarina A.E., Aleksandrova A.V.,
Dogadina A.V., Ionin B.I. – Russ. J. Gen. Chem., vol. 75, No. 1, 5-10, 2005.
39. Krutikov V.I., Erkin A.V., Krutikova
V.V. - Russ. J. Gen. Chem., vol. 82, No. 5, 822-826, 2012.
40. Vetlugina L.A., Nikitina E. T.
Antifungal Polyene Antibiotics [in Russian], Alma-Ata: Nauka, pp. 67-71,
1980.
SUMMARY
V.V.Belakhov1, A.V. Garabadzhiu2a,
B.I.Ionin2b
1Schulich Faculty of Chemistry, Technion –
Israel Institute of Technology Israel; 2aDepartment of Technology of
Microbiological Synthesis and 2bDepartment
of Organic Chemistry, Saint-Petersburg State Technological Institute (Technical
University), Saint-Petersburg, Russia
DIALKYLAMIDOPHOSPHATE DERIVATIVES OF AMPHOTERICIN B:
PREPARATION AND ANTIFUNGAL ACTIVITY
The chemical modification of the heptaene macrolide antibiotic
amphotericin B with dialkylphosphites was carried out in the conditions of
Todd-Atherton reaction. It was shown that reactions of amphotericin B with different
dialkylphosphites resulted in the formation of its corresponding
dialkylamidophosphate derivatives. Physicochemical properties of
dialkylamidophosphate derivatives of amphotericin B and their antifungal
activity against Candida yeast-like fungal strains were studied.
Keywords: amphotericin B, dialkylphosphites, derivatives, antifungal activity.