Химия и химические технологии/ 5.Фундаментальные проблемы создания новых материалов и технологий
Ph.d.
Student, Sadeq Muneer Shawkat
National technical university "Kharkov polytechnic institute",
Ukraine
Technical aspects of biodiesel
production by using a heterogeneous catalyst
The
reactions for direct transformation of vegetable oils into ethyl esters and
glycerol have been known for more than a century. The reactions of interest
today, mainly those producing ethyl esters from rapeseed, soybean and sunflower
oils, have been studied and optimized in order to manufacture the high quality
diesel fuel known as biodiesel. With
over ten years of development and commercial use in
Europe, biodiesel has now
proved its value as a fuel for diesel engines [1-2]. The product is free of
sulfur and aromatics, and, as it is obtained from renewable sources, it reduces
the lifecycle of carbon dioxide emissions higher compared to conventional
diesel fuel. Moreover, recent European regulations in year 2005 have restricted
sulfur content in diesel fuel to no more than 50 ppm [3].
Several
commercial processes to produce fatty acid methyl esters from vegetable oils
have been developed and are available today. These processes consume basic
catalysts such as caustic soda or sodium methylate which form unrecyclable
waste products. This work provides a general description of a new process using
a heterogeneous catalytic system.
Biodiesel
production principles and processes
The
transesterification of triglycerides to ethyl esters with ethanol is a balanced
and catalyzed reaction as illustrated in Figure 1. An excess of ethanol is
required to obtain a high degree of conversion. Rapeseed and soybean oils are among the main vegetable oil candidates
for biodiesel uses.
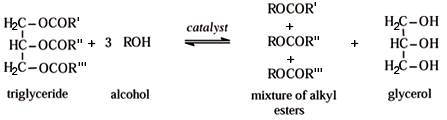
Figure 1. Overall
reactions for vegetable oil alcoholysis.
The
conventional catalysts in natural oil transesterification processes are
selected among bases such as alkaline hydroxides [4]. However,
transesterification could also be performed using acid catalysts, such as
sulfuric, or using metallic base catalysts such as oxides of calcium,
magnesium, or zinc. All these catalysts act as homogeneous catalysts and need
to be removed from the products after the ethanolysis step.
Heterogeneous
catalyzed
To
avoid catalyst removal operations and soap formation, much effort has been
expended on the search for solid acid or basic catalysts that could be used in
a heterogeneous catalyzed process [5-6]. Some solid metal oxides such as those
of calcium, magnesium, and zinc are known catalysts but they actually act
according to a homogeneous mechanism and end up as metal soaps or metal
glycerates. This work a new continuous
process is described, where the transesterfication reaction is promoted by a
completely heterogeneous catalyst. This catalyst consists of a mixed oxide of
zinc and aluminum which promotes the transesterification reaction without
catalyst loss.
The
reaction is performed at a higher temperature than homogeneous catalysis
processes, with an excess of ethanol. This excess is removed by vaporization
and recycled to the process with fresh ethanol. The desired chemical conversion
is reached with two successive stages of reaction and glycerol separation to
displace the equilibrium reaction. The flow sheet for this process appears in
Figure 2.
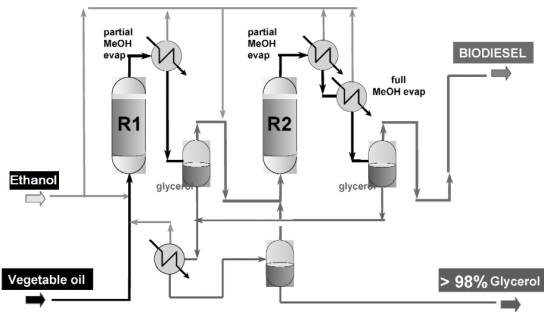
Figure 2. General
scheme for a continuous heterogeneous catalyzed process.
The
catalyst section includes two fixed bed reactors that are fed by oil and
ethanol at agiven ratio. Excess ethanol is removed after each of the two reactors
by a partial flash. Esters and glycerol are then separated in a settler.
Glycerol phases are joined and the last traces of ethanol are removed by
vaporization. Biodiesel is recovered after final recovery of ethanol by
vaporization under vacuum and then purified to remove the last traces of
glycerol. In this heterogeneous
process, the catalyst is very stable with no metal leaching. There is no
formation of either glycerate salts or metal soaps which affords the following
advantages: no neutralization step is required, there is no introduction of
water, and there is no salt formation; these accounts for exceptional glycerol
purity. In addition, there is no waste production of low-value fatty
acids.
The
purity of ethyl esters exceeds 98% with yields close to 100% of the
theoretical. Glycerol treatment is much easier than in homogeneous catalyzed
processes. A simple elimination of ethanol by vaporization suffices and no
chemicals are required. The glycerol produced is neutral, clear and exempt from
any salt, with purities above 98%. This valuable product could be used in many
chemical applications without further treatment. If required, pharmaceutical
grade can easily be reached.
The process feeds are limited to vegetable oils and ethanol and the only
products are biodiesel and a high-purity glycerol that is free of water and
salt. With all its features, the process can be considered as a green process.
Experiments on ethanolysis with acid-containing vegetable oils have also been
conducted with no special acid removal treatment of the raw material.
ethanolysis of a blend of 5% oleic acid in rapeseed oil leads to an ester product composition compatible with
biodiesel requirements. In that case, esterification and transesterification
reactions occur simultaneously.
References:
1. Canakci, M.,
(2007). The potential of restaurant waste lipids as biodiesel feedstocks.
Bioresour. Tech., 98 (1), 183-190 (8
pages).
2.
Biodiesel - A Success Story, Report
of the International Energy Agency, February 2002.
3.
Montagne, X., 2nd European Motor Biofuels Forum, Graz,
September 1996.
4. Kreutzer, U., J. Am. Oil.
Chem. Soc., 1984, 61 , pp.343.
5. Stern, R.; Hillion, G.;
Rouxel, J. J.; Leporq, S. US Patent
5,908,946 (1999)
6. Gelbard, G.; Brès, O.;
Vielfaure-Joly, F. J. Am. Oil. Chem.
Soc.,1995, 72, pp1.