Химия и химические технологии/5.
Фундаментальные проблемы создания новых материалов и технологий
D. t. n.
Melnik A.P., Papchenko V.Y.
National technical university
«Kharkov polytechnic institute»
EMULSIFYING ABILITY OF
AMIDATION PRODUCTS OF SUNFLOWER OIL BY DIETHANOLAMINE
Nowadays due to
development and increasing the range of products in alimentary, cosmetic and
many other branches of industry there is a great increase in a demand as on
nutrient surfactants – natural and modified in chemical way natural substances,
namely monoacylglycerides (MAG), so and on diethanolamides of fatty acids
(DFA). DFA, and surfactants, show surface activities and are used in cosmetic
preparations for foam stabilization, viscosity increasing, softening an action
of washing preparations on the human skin [1], and join the compositions of
cosmetic formulations and products as emulsifiers [2, 3]. In earlier works [4, 5] the possibility of simultaneous production of
these surfactants classes has been stated.
For the moment of now nothing
is known about surfactant properties of DFA mixed with MAG and diacylglycerides
(DAG) obtained by amidation reaction of sunflower oil (SO) by diethanolamine
(DEA).
The aim of this work is
to research the emulsifying ability of products obtained by amidation reaction
of SO by DEA.
Obtaining the reactive
masses has been carried out, as described previously elsewhere [6]. For
investigation the reactive masses obtained in molar ratios (MR) SO:DEA 1:2, 1:3
and temperatures 433 – 473 К have been used. Emulsifying ability has been
determined according [7] by measuring the changes of emulsion volume
“water-hydrocarbon” and “water-oil” in the time in the presence of synthesis
products in concentrations 0,25 – 1 % compared with sodium
oleate in the same concentrations at the temperature 293 К. As non-water phase during
emulsions production tetradecane and SO have been used.
According the data obtained the kinetic curves (Fig. 1 – 4) for emulsion destruction have been built out, on the basis of ones the emulsion stability (ES) has been estimated.
|
а |
|
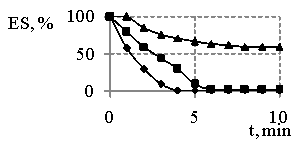
Fig. 1
– The dependence of emulsion
stability (ES) water-hydrocarbon in the presence of sodium oleate on the time
(a) and reaction products obtained at MR SO:DEA 1:2 and synthesis temperature
473 K (b) on the time, where
sodium
oleate concentration ![]() - 1 %,
- 1 %, ![]() - 0, 5 %,
- 0, 5 %, ![]() - 0,25 %;
- 0,25 %;
concentration
of reaction products ![]() - 1%,
- 1%, ![]() - 0, 5 %,
- 0, 5 %, ![]() - 0,25 %
- 0,25 %
|
|
|
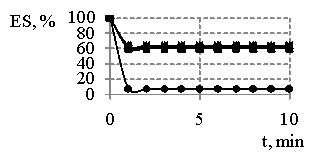
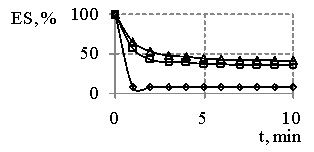
Fig. 2
– The dependence of emulsion
stability (ES) “water-oil” in the presence of reaction products obtained at MR
SO:DEA 1:2 and synthesis temperature 443 K (a) and reaction products
obtained at MR SO:DEA 1:3 and synthesis temperature 433 K (b) on the time,
where concentration of reaction products
![]() - 1%,
- 1%, ![]() - 0, 5 %,
- 0, 5 %, ![]() - 0,25 %
- 0,25 %
On the basis of the results (Fig. 1) we can clearly see the products resulted and obtained at MR SO:DEA 1:2 are more effective emulsifiers than sodium oleate. It is necessary to point out (Fig. 1, 2) the fact that the emulsions tend to be destabilized in the presence of sodium oleate immediately after their having been obtained, and the emulsion destabilization in the presence of synthesis products is characterized and featured by the dependences, whereas on some of them the gaps with 100 % stability have been determined. Besides, the increase in emulsifiers concentrations and temperatures provides the increasing stability of emulsions.
|
а |
b |
|
c |
|
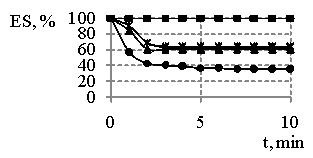
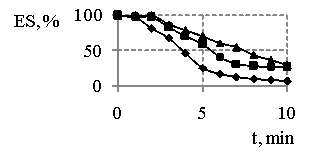
Fig. 3 – Changes in emulsions
stability (ES) “water-hydrocarbon” in the presence 0,25 % (а), 0,5 % (b), 1 % (c) of
products concentrations obtained at MR SO:DEA 1:3 in the dependences on time and
temperature of synthesis, where
![]() - 433 К,
- 433 К, ![]() - 443 К,
- 443 К, ![]() - 453 К, х - 463 К, * - 473 К,
• - sodium oleate
- 453 К, х - 463 К, * - 473 К,
• - sodium oleate
The stability of
emulsions where the emulsifiers are the products obtained at MR of reagents 1:3
(Fig. 3) is changed not only in the time, but also accompanied with the
changes in the temperature of synthesis and concentrations. If at 0,25 % concentration of the synthesis products(Fig. 3a) the
emulsion stability nearly doesn’t depend on the temperature, so the increase in
the temperature of synthesis until 443 K leads to the substantial
increasing of it. As the concentration of reaction products increases
0,5 % – 1 % (Fig. 3b, c), so we have observed the
influence of the temperature. Products, obtained at higher temperatures,
provide for the first time the production of emulsions differ in their
stability, but with following of time their stability becomes equal and is on
the level in 60 %, that keeps stable during long time while using all the
concentrations studied out as in the case of using sodium oleate.
In the contrast of them,
emulsions with usage of products obtained at lower MR of reagents have been
destabilized more quickly. This has been confirmed by results shown in
Fig. 1-3. It’s necessary also to point out that products obtained at MR of
reagents as 1:3 will in any cases from more stable emulsions compared with
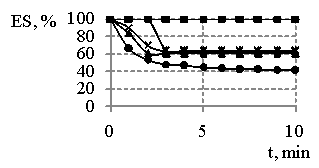
emulsions formed in the presence sodium oleate. The comparing of emulsification
ability of three emulsifiers (Fig. 4) has shown the fact that the
conditions of synthesis could regulate stability of emulsions.
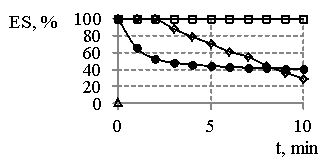
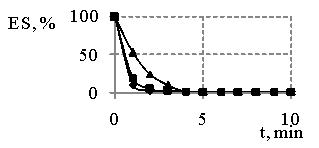
Fig. 4
– The comparing of changes in emulsions stability (ES) in the 1 %
concentration of products obtained and sodium oleate the time,
where ![]() - MR 1:3,
- MR 1:3, ![]() - MR 1:2, • - sodium oleate
- MR 1:2, • - sodium oleate
One of the reaction
products has been used as emulsifiers in the formulation of cosmetic
preparation, namely cream for hands care with following content, % wt: was
– 4,1; glycerol – 4,1; vegetable oil – 36,1; reaction products – 5,2; sodium
tetraborate – 1,0; distilled water – 49,5. It has been stated according [7],
that cream obtained is an inverse emulsion “water in the oil”. The investigation
of cream stability according [8] that has been defined on the bases of the
quantity of oil phase releases in the time has indicated about the fact the
cream obtained is a stable inverse emulsions.
Conclusions:
1. It is determined
the products of sunflower oil amidation possess the surfactant features showing
emulsifing ability.
2. Stability of
emulsions “water-hydrocarbon” and “water-oil” in the presence of synthesis
products could be regulated by synthesis conditions. Emulsions obtained are in
some cases more stable comparing with emulsions obtained in the presence of
sodium oleate.
3. It has been
shown that amidation products from sunflower oil could be used as emulsifiers
for preparation of cosmetic cream formulatios.
References:
1. Плетнёв М.Ю. Косметико-гигиенические моющие средства / М.Ю. Плетнёв – М. : Химия, 1990. – 272 с. 2. Пат. 2279870 Россия, МПК А61К8/73, А61К8/34, А61К8/19, А61К8/29, А61К8/25, А61К8/37, А61Q19/00. Средство для защиты кожи рук / Кузьмин В.Н., Кузьмина Р.М., Машина Л.С.; ООО “РМ”, Капралова Л.М. – №2004134665/15; заявл. 18.11.2004; опубл.20.07.2006. 3. Окрашивающие составы, содержащие пиразолопиримидин в качестве основы для окисления и 6-алкокси-2,3-диаминопиримидин как агент сочетания. ЕПВ, МКИ7 А61К7/13 Kravtchenko Sylvain, Lagrange Alain – Заявл.05.04.2004; опубл. 19.10.2005. // Химия: РЖ, 2007. – 19Р2.39П. 4. Дослідження одержання азото-, кисеньвмісних похідних жирних кислот амідуванням соняшникової олії діетаноламіном / А.П. Мельник, В.Ю. Папченко // Вісник національного технічного університету “Харківський політехнічний інститут”. – 2010. – № 4 – С. 3–6. 5. Дослідження амідування триацилгліцеринів соняшникової олії діетаноламіном / А.П. Мельник, В.Ю. Папченко, Т.В. Матвєєва // Вісник національного технічного університету “Харківський політехнічний інститут”. – 2007. – № 27. – С. 92–95. 6. Дослідження реакції утворення алкілкарбон-N-(дігідроксіетил)амідів / А.П. Мельник, В.Ю. Папченко, Т.В. Матвєєва [та ін.] // Вісник Національного технічного університету “Харківський політехнічний інститут” – 2003. – № 11. – С. 64-69. 7. Мельник А.П. Практикум з хімії та технології поверхнево-активних похідних вуглеводневої сировини : навчальний посібник [для студ. вищ. навч. закл.] / Мельник А.П., Чумак О.П., Березка Т.О. - Харків: Курсор, 2004. – 277 с. 8. ГОСТ 29188.0-91 Изделия парфюмерно-косметические. Правила приемки, отбор проб, методы органолептических испытаний.
 а
а