Химия и химические технологии/8. Кинетика и катализ
Zaitseva A.S., Arlyapov V.A., Turovskaya A. D.
Tula State University
Determination of the rate constants
for the interaction of D. hansenii
cells with electron transport mediators
Mediator transfer is
widely used for the development of bioanalytical bioelectrochemistry sensors,
biofuel cells, etc. As the electron acceptor, a mediator transports electrons
from the active center of the biomaterial to the indicator electrode. The
effectiveness of this process will depend on the speed of interaction between
the mediators and used biomaterial, this way, the aim of this work is to
determine the rate constants for the interaction of D. hansenii cells with electron transport mediators. As mediators
following compounds were used: thionine, methylene blue, neutral red and
2,6-dichlorophenolindophenol. Using them as mediators caused by the fact that
they are non-toxic and capable of transferring electrons from the enzyme active
site of the cells to the electrode surface. D.
hansenii yeast cells have been used as microorganisms, as characterized by
a wide range of oxidizable substrates and enzyme systems in stable stress
conditions.
Process mediator
interaction with the yeast cells is described as follows: substrate penetrates
through the outer cell membrane and interacts with the D hansenii enzyme, resulting enzyme is recovered and, in turn,
gives electrons mediator molecule directly or through a specific site of the
respiratory chain. In this case we can speak about bisubstrate reaction which
proceeds according to the mechanism of "ping-pong" when the
successive steps of reacting the substrates with the active site of the enzyme
are separated by an irreversible chemical transformation that can be described
by the following scheme:
![]()
![]()
where S and P - substrate and product; MOK
and MB - oxidized and reduced form of the electron acceptor;
EOK and EB - enzyme,
located in the cytoplasmic membrane of the bacterial cell in the oxidized and
reduced form, respectively;
k1, k-1, k2, k3,
k-3 and k4 - the rate constants corresponding reaction
steps.
The general equation of bisubstrate enzymatic reaction speed that
occurs by the mechanism of "ping-pong" can be written as:
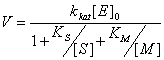
where [S] and [M] - concentration of mediator
and substrate, respectively;
[E] - the concentration of the enzyme complex
in vivo;
kkat - catalytic constant of the
reaction;
KS and KM - Michaelis
constant for the substrate and a mediator, respectively.
With an excess of substrate and low
concentrations of the mediator [M] << KM the equation can be
simplified and find rate constants for the interaction of cells with mediators
kOx (Karyakina E.E, 2009):
![]()
All measurements were performed using the SF
103. Kinetic studies were carried out at a wavelength corresponding to the
maximum absorption of the mediator oxidized form: 660 nm (methylene blue), 520
nm (neutral red), 600nm (2,6-dichlorophenolindophenol), 600 nm (thionine). From
the change in optical density was determined the rate of recovery of the
mediator by D. hansenii cells over
time, as the slope, taking into account the extinction coefficient. The
obtained dependences of kinetic constants were found biochemical mediators
recovery rate, which are shown in Table 1.
Table 1. Rate constants for the interaction of D. hansenii cells with electron
transport mediators.
|
Biomaterial / mediator/ substrate |
Rate constant, (s-1) |
|
Yeast D. hansenii/ thionine/ glucose (0,05 мМ) |
0,014±0,004 |
|
Yeast D. hansenii/ methylene blue/ glucose (0,05мМ) |
0,007±0,001 |
|
Yeast D. hansenii/ neutral red/ glucose (0,05мМ) |
0,018±0,003 |
|
Yeast D. hansenii/ 2,6-dichlorophenolindophenol/ glucose (0,05мМ) |
0,008±0,002 |
According to the rate constants of interaction
the mediators with D. hansenii yeast,
we conclude that the most effective mediator is a neutral red, as in this
system the rate constant is maximum.
This work was
supported by the grant of the President of the Russian Federation (contract № 14.Z56.16.5425-МК)
and by the grant of the Russian Foundation for Basic Research (contract №
16-48-710959 р_а)
References:
Karyakina E.E., Vokhmyanina D.V., Sazontova T.G., Sabitov A.N., Borisova A.V., Arkhipenko Yu.V., Tkachuk V.A., Zolotov Yu.A., Karyakin A.A. Kinetic approach for evaluation of total antioxidant activity in food samples // Talanta. 2009. V. 80. P. 749–753.