Ìatysina Z.A., Botsva N.P., Elina E.V., Yarochevskaya A.V.
Oles Honchar
Dnipropetrovsk national university, Ukraine
THEORETICAL STUDY OF HYDROGEN-SORPTION
PROPERTIES OF FULLERITE C60
In terms of the increase in energy needs of humanity
and the background of the deterioration of the planet's ecological situation is
urgent search for alternative and clean energy. One of these sources can be
hydrogen burning which is not accompanied by the release of hazardous products.
It is known that reversible hydrogen sorbent that can be used for hydrogen
energy needs can be fullerite F = C60 [1,2].
The subject of interest of the present paper is the theoretical study of the statistical
theory of lattice hydrogen solubility in fullerite C60 based
on the distribution of hydrogen atoms in the interstices of four various types.
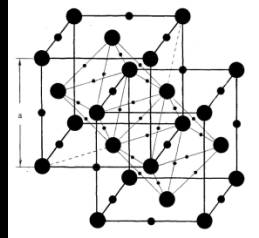
Under normal conditions fullerite C60 has a
face-centered cubic (fcc) lattice containing 4 units of fullerene
molecules and 4 octahedral (O),
8 tetragonal (θ), 32 trigonal (Q) and 36 bigonal (D) interstices,
where assumed to be
placed hydrogen atoms (Fig. 1) [3].
|
|
|
|
a |
b |
|
Fig.1.
Face-centered cubic unit cell of hydrofullerite C60Hx |
|
|
|
position of
fullerene molecules and hydrogen in octahedral |
|
and bigonal (a) and tetragonal
and trigonal (b) interstitial
lattice |
|
The number of fullerene molecules in the immediate vicinity is hydrogen
for different types of interstitial respectively as follows: 6 for O, 4 for θ, 3 for Q and 2
for D. Distances between adjacent pairs of fullerene-hydrogen (FH) have the following
values (in nanometers):
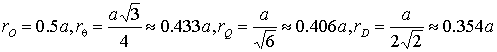 , (1)
, (1)
where
![]() – the fcc lattice parameter of fullerite [3].
– the fcc lattice parameter of fullerite [3].
For calculation of free energy ![]()
![]() (2)
(2)
introduce
the signs ![]() – the number of crystal (fullerene) clusters;
– the number of crystal (fullerene) clusters;
![]() ,
, ![]() ,
, ![]() ,
, ![]() – the number of interstices O, θ, Q, D;
– the number of interstices O, θ, Q, D;
![]() ,
, ![]() ,
, ![]() ,
, ![]() – the number of
hydrogen atoms in interstices O, θ, Q, D, with
some of interstices can be found vacant.
– the number of
hydrogen atoms in interstices O, θ, Q, D, with
some of interstices can be found vacant.
The full number of the
hydrogen atoms in all interstices is
![]() . (3)
. (3)
The concentrations of the hydrogen atoms in interstices O, θ, Q, D with
respect to the fullerene number are
![]() ,
, ![]() ,
, 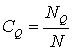 ,
, ![]() . (4)
. (4)
The full
concentration of the lattice hydrogen atoms in fullerite is
![]() . (5)
. (5)
The hydrogen atom energy in each interstice is defined
by a sum of energies of it’s interaction with the
nearest fullerenes
![]() ,
, ![]() ,
, ![]() ,
, ![]() , (6)
, (6)
where
![]() ,
, ![]() ,
, ![]() ,
, ![]() (7)
(7)
– the
energy of pair interaction of hydrogen atoms with the nearest fullerenes for
interstices O, θ, Q, D.
Configurational energy E
and thermodynamical probability W will be equal
![]() (8)
(8)
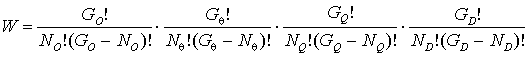 (9)
(9)
Substituting (8) and (9) into
(2) taking into account the Stirling formula, equitable for the big numbers,
find the free energy in the form
![]()
![]() (10)
(10)
![]()
Equilibrium states of hydrofullerite are defined from the clause of free energy minimization which is easily to find with
Lagrange's method of undetermined multipliers
![]() ,
, ![]() ,
,
![]() ,
, ![]() ,
(11)
,
(11)
where ![]() is the chemical
potential, defining the activity of hydrogen atoms, which can differ from
interstices O, θ, Q, D.
is the chemical
potential, defining the activity of hydrogen atoms, which can differ from
interstices O, θ, Q, D.
Each formula, taken separately from (11), defines the temperatur
dependence of hydrogen solubility in the interstices of four various types.
Character of the temperature dependence each of the concentrations is difined
also with energy's parameters ![]() ,
, ![]() ,
, ![]() ,
, ![]() , which may be of different signs in the case of
the different distances (1) in the couples of FH.
, which may be of different signs in the case of
the different distances (1) in the couples of FH.
Fig.2 shows the monotonous temperature dependence of a concentration (11)
for positive and negative energy values ![]() ,
, ![]() ,
, ![]() ,
, ![]() . If Ui > 0 solubility increases and if Ui
< 0 solubility decreases whis the temperature rise.
. If Ui > 0 solubility increases and if Ui
< 0 solubility decreases whis the temperature rise.
When comparing the calculation results with the experimental data [4]
there is a quality matching the temperature dependences of the solubility of
hydrogen in fullerite.
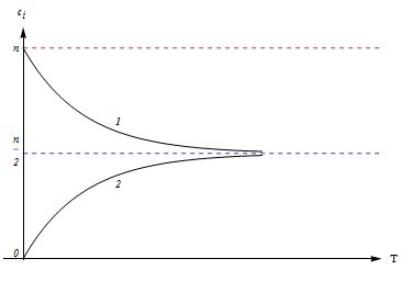
Fig. 2. Temperature dependence of a hydrogen solubility Ñi
in the
interstices of four various types: 1 – Ui > 0; 2 – Ui < 0;
n = 1, 2, 8, 9 for i = O,
θ, Q, D
Thus the statistical theory of solubility of hydrogen in the lattice of
fullerite C60 considering the distribution of hydrogen atoms in the interstices of four
types is developed. Calculated free energy hydrofullerite and established conditions of thermodynamic
equilibrium system, which found the concentration of hydrogen atoms in all the
interstices and identified similar to the experimental temperature dependence
of the solubility of hydrogen atoms when placed in the interstices of various
types.
References
1. Fullereny – osnova materialov
budushchego / V.I.Trevilov, D.V.Shchur i dr. – K.: ADEF-Ukraina, 2001. – 48 p.
2. Atomarnyye, fullerenovyye i
drugiye molekulyarnyye fazy vnedreniya / Z.A.Matisina, D.V.Shchur, S.Yu.Zaginaychenko.– D.: Izd-vo
Makovets'kiy, 2012. – 888 p.
3. Rastvorimost' primesey v
metallakh, splavakh, intermetallidakh, fulleritakh / Z.A.Matisina, S.Yu.Zaginaychenko, D.V.Shchur.
– D.: Nauka i obrazovaniye, 2006. – 514 p.
4. Savenko A.F. Osobennosti
gidrirovaniya fullerita C60. Avtoreferat kand. diss. – K.:
KIM, 2013. – 20 p.