Myrzahanova
M.N.
Kokshetau
State University named after Sh. Ualikhanov
The
influence of vasoactive substances on the contractive activity of the smooth
muscle cells of the rats’ and ground squirrels’ lymphadens in ontogenesis
The doctrine of lymphology
appeared long ago (already as early as in 1627, Azelli); and its functional
importance for the vital activity of the living bodies is unquestionable. As a
proof we may just mention such a fact that the lymphatic system plays a very
important role in forming antibody-mediated and cell-mediated immunity[1] and
that many pathologic processes spread through the lymphatic viae[2].
Regional lymph
nodes are one of the first to react in case of inflammation and to detain microorganisms
and tumor cells which favor the formation of clumps and retention of a disease agent[3].
That is why a great
importance is attached to the problem of studying the functions of lymph nodes
and vessels. However, we should mention, that the problem of lymph nodes
motility has not been sufficiently investigated yet. This fact seems likely to
be connected with the conventional view about the inactivity of lymph nodes and
with the understatement of their energy, accumulated by the nodes themselves
due to the presence of the lissosphincter and nerval elements and their aptitude
for the contractive activity[4-6].
Lymph nodes hold a
unique position in the lymphatic system due to the functional peculiarities
characteristic for the organs of the immune system. The presence of the fatty
tissue must be regarded as an essential structural environment for the lymph
nodes. Fatty tissue is a specific formation which prepares place for the lymph
nodes, being the buffered, protective link of other structural formations[7].
In case of stress
there happens redistribution of T-lymphocytes in the direction of the regional
lymph nodes of the limbs and skin integument[8].
Today the study of
the structure and functions of the lymph nodes is of a great interest for
morphologists, physiologists, immunologists, hematologists, clinical physicians
and other experts as the functions performed by lymph nodes play a great role
in the vital activity of the organism.
Thymus became the
central organ of the immunity system for fish[9]. There were conducted
investigations to define the role of hypothalamus and hypothalamic peptides of
gonadrophin releasing hormone and beta hypophamine in the process of the immune
system formation and functioning in the ontogenesis of rats. It turned out that
gonadrophin releasing hormone proceeds to the control actions of the
immunologic functions as early as in the prenatal ontogenesis. In the thymus of
the fetuses there were found gonadrophin releasing hormones – immuno-positive
cells, morphologically similar to thymocytes. The synthetic process of
gonadrophin releasing hormone in the thymus is controlled by hypothalamus. The
data give us an opportunity to conclude that beta hypophamine controls the work
of the immune system in the course of the whole life of animals[10].
The results of the
research on morphology and physiology of the lymphatic system are reflected in
the works of the researches both in our country and abroad[11-17].
Physiologists investigated the contraction of
lymph nodes when reacting to electric stimulation (Myrzakhanov N.M., 1987) or
injection of pharmacological agents[18].
For the first time
the aptitude of the lymphatic nodes for the rhythmic spontaneous contractions
was shown in the work of Myrzakhanov N.M. in 1987. In this work the author suggested
and registered a classification of forms of the contractive activity and their
frequency-amplitude characteristics.
The given fact makes
us reconsider our views on the role of lymph nodes in the liquid motion in the
organism. The fact that a great number of visceral lymph nodes can be found in
the main transporting lines of the lymph proves their great role in the process
of liquid transporting.
The lymphatic
system, being a part of the cardiovascular system in the organism, is involved
in sustaining homeostasis, removing from the telae surplus liquid and albumins
which passed to the interstitial space having left the blood-vascular
system[19,20]. Due to the literature on the subject we learn that denaturated
proteins and ferments are the first to enter lymphatic capillary tubes (Lindena
E.A., 1979; Sviridkina, 1989), and that the quantity and structure of the lymph
changes, while the lymph is passing through the lymphatic vessels and
nodes[21,22].
Lymph nodes perform
the following functions: lymphocytopoiesis, barrier and filtration functions,
transportation, immunopoiesis, metabolism, deposition (especially of the
vitamins A and B), internal secretion (heparin), erythrocytoschisis. It is
natural to suppose that to understand better the processes of performing the
above mentioned functions of the lymph nodes it is necessary to study the
generation of the spontaneous lymphatic contractive function of the lymph
nodes, in postnatal ontogenesis, in particular.
Research methodology
To carry out the
experiment there were taken as many as 60 laboratory rats and 40 ground
squirrels. There were taken from the rats 17 isolated specimens of
submandibular, axillary, inguinal, cardiac, intestinal, mesenterial, nephritic
and hepatic lymph nodes. From the ground squirrels there were taken 7 isolated
specimens of submandibular, axillary, inguinal, cardiac, intestinal,
mesenterial, nephritic and hepatic lymph nodes. Physical inactivity of the
animals was secured by means of ether-chloroform anaesthesia.
In sum, there were carried out 820
experiments with the isolated vascular specimens of the nodes. Spontaneous rhythmic
contractions of the lymph nodes were made on the 1, 10 and 30 day after the
birth of rat litter and on the 1, 15, 30, 90 day after the birth of ground
squirrels’ litter. In the course of the experiment with the rats’ and ground
squirrels’ isolated lymph nodes their contractive activity was explored;
prosected nodes were placed into thermostatically controlled chamber with the
flowing solution of Krebs; tracing of the contractive activity was done with
the help of the mechanotron 6Mx1C, in accordance with the established procedure,
graphical recording being done on recording meter H-327-5 (Orlov and others,
1975; Luchinin, 1979; Myrzakhanov, 1987), on the unit modified by Hanturin in
1996. There were used longitudinal specimens of the lymph nodes, from 5 to
10-12 mm in length. One end of the longitudinal specimens of the nodes was
fixed to the bottom of the chamber of the vertical type or to the sidewall of
the horizontal type, the other end was attached to the force sensing device
(mechanotron for the sensitive type 6Mx1B). For the isolated lymph nodes of the
rats and ground squirrels there was used the solution of Krebs of the following composition: NaCl –
124,0; NaH2PO4 – 1,2; RCl – 5,9;
CaCl2-2,5; MgCl2 – 1,2; NaHCO3 – 15,5; C6 H12 O6 – 11,5 mol/litre of
the distilled water. In the course of work there were used solutions with pH
7,2-7,3, at the temperature of 37° C. Nutrient
solutions were oxygenated with the gas
mixture: 95% O2 and 5% CO2. The following
physiologically active substances were employed as irritators for the nodes:
adrenalin hydrochloride, acetyl chlorine chloride, noradrenalin.
Contractive
activity of the isolated lymph nodes was registered on the graph paper of the
potentiometer KSP-4 or milliampervoltmeter of the tracer H327-5. During every
experiment there were provided conditions for life sustaining of the isolated
nodes during each experiment there was followed a certain order of making
intervals of agents injecting and the order of the substances washing off.
The results of the research.
The results of the
carried out experiments showed that the character of the spontaneous rhythmic
contractive activity of rats’ and ground squirrels’ lymph nodes differs considerably
in accordance with the duration of the postnatal ontogenesis of an organ.
A number of
experiments were aimed at analyzing the influence of the vasoactive substances
– adrenalin, noradrenalin and acetylcholine, - on the contractive activity of
the isolated specimens of the lymph nodes typical for the representatives of
mammals in ontogenesis. The results of the research showed that the quantity of
adrenalin on the first day in submandibular, axillary, inguinal, mesenterial,
intestinal, hepatic, cardiac and nephritic lymph nodes was from 0,65±0,027 ml/min to
1,78±0,204 ml/min for
the rats and from 0,18 ± 0,0045 ml/min for the ground squirrels; on the tenth day –
from 0,87 ± 0,055 ml/min to
2.33±0,341 ml/min for
the rats and from 1,20±0,0475 ml/min for
the ground squirrels; on the thirtieth day from 0,57±0,031 ml/min to 2,15±0,299 ml/min for
the rats and from 1,71±0,0410 ml/min for
the ground squirrels; and on the ninetieth day from 1,03 ± 0,0247 ml/min for
the ground squirrels in comparison with the initial level. The minimal
effective doze was that in mesenterial on the first day for the rats and
hepatic for the ground squirrels, the maximal was on the tenth day in axillary
and on the thirtieth in the inguinal lymph nodes of the postnatal development. The quantity of noradrenalin on the first day
was from 0,50±0,086 ml/min for
the rats and from 0,03 ± 0,0008 ml/min for the ground squirrels; on the tenth day –
from 1,75 ± 0,111; on the
fifteenth day from 1,81±0,0432 for the
ground squirrels; on the thirtieth day from 1,08±0,075 ml/min for the rats and
from 1,71±0,0410 ml/min for
the ground squirrels; and on the ninetieth day from 1,03 ± 0,0247 ml/min for
the ground squirrels in comparison with the initial level. On the first day the
minimal effective doze was that in the cardiac lymph nodes for the rats and
ground squirrels, the maximal was on the tenth and thirtieth day in the axillary
lymph nodes of the rats and ground squirrels of the postnatal development. The
quantity of acetylcholine on the first day was from 1,21±0,092 ml/min for
the rats and from 0,08 ± 0,0020 ml/min for the ground squirrels; on the tenth day –
from 1,75 ± 0,111 for the
rats; on the fifteenth day from 1,92±0,0457 for the
ground squirrels; on the thirtieth day from 2,09±0,280 ml/min for the rats and
from 1,71±0,0410 ml/min for
the ground squirrels; and on the ninetieth day from 1,22 ± 0,0301 ml/min for
the ground squirrels in comparison with the initial level. On the first day the
minimal effective doze was that in the nephritic lymph nodes for the rats and
in the mesenterial lymph nodes for the ground squirrels, the maximal was on the
tenth day in cardiac and inguinal for the rats, and on thirtieth day in the
intestinal lymph nodes of the ground squirrels of the postnatal development.
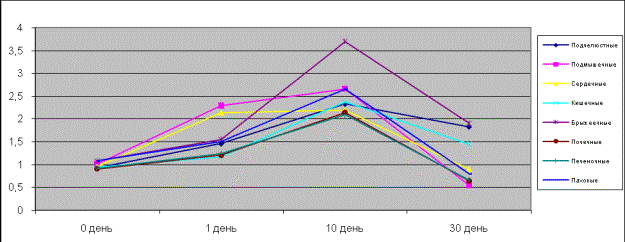
In pictures 1, 2
one can see the contractive activity in the isolated clavicular, axillary,
inguinal, nephritic, hepatic, intestinal, mesenterial and cardiac lymph nodes
of the rats and ground squirrels when acting on adrenalin. It is seen that the
isolated specimens of the rats’ hepatic lymph nodes on the first day were of a
greater affinity to adrenalin and more responsive than other lymph nodes. On
the tenth day the isolated specimens of the axillary, submandibular,
intestinal, inguinal, mesenterial, nephritic lymph nodes were more sensitive
and responsive than cardiac ones, and on the thirtieth day there was observed
rats’ hepatic lymph nodes fall-off. On the fifteenth and thirtieth days ground
squirrels’ isolated specimens of hepatic, nephritic, mesenterial and intestinal
lymph nodes appeared more sensitive but the inguinal nodes were more responsive
than axillary, submandibular and cardiac.
Picture 1.
Contractive activity of the isolated lymph nodes of rats when acting on
adrenalin
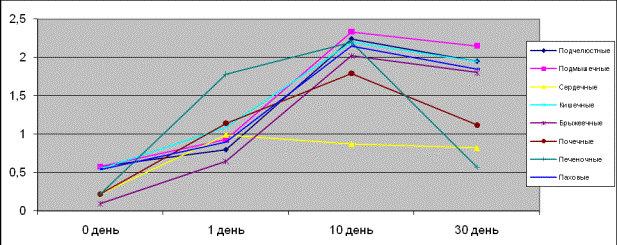
Picture 2.
Contractive activity of the isolated lymph nodes of ground squirrels when
acting on adrenalin
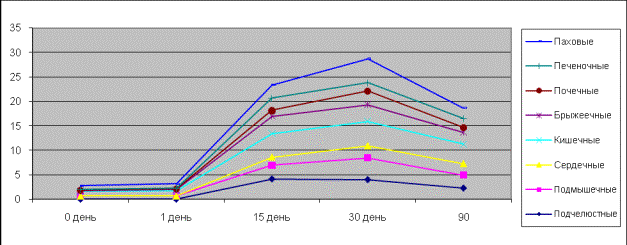
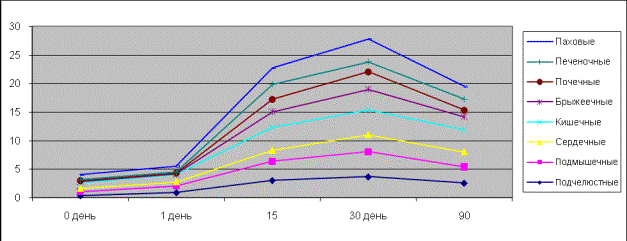
In pictures 3, 4
one can see rhythmic contractions influenced by noradrenalin in the isolated
specimens of the clavicular, axillary, inguinal, nephritic, hepatic,
intestinal, mesenterial and cardiac lymph nodes of rats and ground squirrels.
It is clear from the picture that affineness of smooth muscle cells of the
inguinal lymph nodes to noradrenalin differs from that of the submandibular,
axillary, mesenterial nodes and was much higher in the inguinal and intestinal
lymph nodes on the tenth day of the ontogenetic development. Isolated specimens
of the submandibular, axillary, mesenterial lymph nodes turned out to be the
most sensitive to noradrenalin and responded in dose-depended tonic
contractions of the rats. On the fifteenth and thirtieth days the isolated
specimens of hepatic, nephritic, mesenterial and intestinal lymph nodes were
more sensitive, but the inguinal nodes were more responsive than axillary,
submandibular and cardiac nodes.
Picture 3.
Contractive activity of the isolated lymph nodes of rats influenced by
noradrenalin.
Picture 4.
Contractive activity of the isolated lymph nodes of ground squirrels influenced
by noradrenalin.
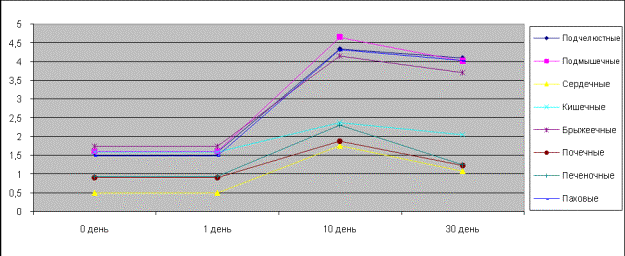
In pictures 5, 6 one can see spontaneous
contractions influenced by acetylcholine in the isolated specimens of the
clavicular, axillary, inguinal, nephritic, hepatic, intestinal, mesenterial and
cardiac lymph nodes of rats and ground squirrels. As it is seen from the
picture, the most sensitive to acetylcholine in the comparison list of the
investigated lymph nodes were the isolated specimens on the first day of the
postnatal development of axillary and cardiac nodes. On the tenth day of the
ontogenetic development rats’ mesenterial nodes are very much responsive, and
on the fifteenth and thirtieth day ground squirrels’ inguinal lymph nodes are
very much responsive.
Picture 5.
Contractive activity of the isolated lymph nodes of rats influenced by
acetylcholine
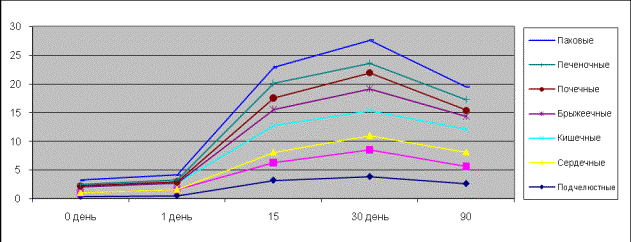
Picture 6.
Contractive activity of the isolated lymph nodes of ground squirrels influenced
by acetylcholine
Our material proved
that the contractive responses of the lymph nodes to the adrenalin,
noradrenalin and acetylcholine agency are caused by the participation of a- adrenergic
receptors; b- adrenergic
receptors to a certain degree reduce the influence of a- adrenergic receptors on the
contractions. As it is seen from the pictures the formation trends of the
frequency and amplitude characteristics, constituting the spontaneous
contractive activity of lymph nodes in the postnatal development of rats and
ground squirrels, have analogous forms. By the moment of birth and on the
ensuing 10-30 days, and for certain lymph nodes up to the 15th day
(cardiac, axillary, submandibular) the level of the spontaneous contractive
activity of all the analyzed lymph nodes is either very low or almost missing,
the observed contractions are irregular and spontaneous, without any visible
regularity. On the tenth day after the rats’ birth and on the fifteenth day
after the ground squirrels’ birth one can notice a very high level of the lymph
nodes contractive activity, with the esception of the rats’ cardiac nodes
influenced by adrenalin, noradrenalin (by the amplitude), and the ground
squirrels’ submandibular nodes influenced by adrenalin, noradrenalin and
acetylcholine (by the amlitude and frequency). One may observe regularity of
all components of the lymph nodes contractive activity and the synchronous
increasing of the total level of the electrobiological activity. In the ground
squirrels’ inguinal, hepatic, nephritic, mesenterial and intestinal nodes on
the 15th and 30th day (by the amplitude) one may observe
upward movement of the specific curve of lymphonodullogram, peculiar for the
organs, and its further decrease on the 90th day of the postnatal
development. It is proved that in the course of the whole postnatal period of
life ground squirrels’ frequency-amplitude characteristics of the lymph nodes
contractive activity change unidirectionally, i.e. synergistically.
Thus, the
activation of the given animals’ lymph nodes spontaneous contractive activity
occurs between the 1st, 10th days after the birth for the
rats and 15th, 30th days for the ground squirrels; a
certan decrease of this activity falls on the 30th and 90th
days of the postnatal development. In the same intervals of time there was
observed increasing of the total level of the lymph nodes electrobiological
activity.
Conclusion
The activation of
the spontaneous contractive activity of the lymph nodes of the soma and
internals occurs twice – between the 1st, 10th days, and
also on the 15th and 30th days after the birth with the
ensuing decrease of this activity on the 30th and 90th
days of the postnatal ontogenesis.
Literature:
1. Жданов Д.А. Общая
анатомия и физиология лимфатической системы. Л.,1952
2. Сапин М.Р., Юрина Н.А.,
Этинген Л.Е. Лимфатический узел (Структура и функция). –М.,1978. –С.272.
3. Поликар А. Физиология и
патология лимфоидной системы. М.,1965
4.
Булекбаева
Л.Э. Роль корковых структур головного
мозга и мозжечка в регуляции лимфообращения. Алма-Ата, 1974
5.
Мырзаханов
Н. Рефлекторные влияния на ток, белковый и ионный состав кишечной лимфы //
Автореферат дисс. канд. биол. наук. –Таллин, 1974. –С. 16.
6.
Жданов
Д.А., Володько Н.С. Схема интрамуральной иннервации ductus thoracicus человека. – «Архив анатомии, гистологии,
эмбриологии», 1968, т.54,№3, с.64-68
7.
Шведавченко
А.И., Русских Т.Л., Рыбакова Л.И. Особенности анатомии и топографии
лимфатических узлов // Материалы I Сибирского съезда лимфологов с международным
участием «Проблемы экспериментальной, клинической и профилактической
лимфологии». – Новосибирск, 2006. – С.361.
8.
Б.Б.
Фукс. Перераспределение лимфоцитов в
организме с задержкой в лимфоузлах под влиянием антигенов и стресса // III
– международный конгресс «Эндоэкологическая медицина». Кипр, 2007. С.15
9.
Полевщиков
А.В., Дьячков И.С., Кудрявцев И.В. Становление структуры и функций тимуса:
сравнительно-иммунологический анализ // Научные труды I Съезда физиологов СНГ.
– Сочи, Дагомыс, 2005. – С.104.
10. Захарова Л.А.,
Мельникова В.И., Попова Н.А., Хегай И.И., Иванова Л.Н., Кольцова Н.К.
Функциональное значение гипоталамических пептидов гонадотропин-рилизинг гормона
и вазопрессина в регуляции иммунного ответа в онтогенезе крыс // Научные труды
I Съезда физиологов СНГ. – Сочи, Дагомыс, 2005. – С.102.
11. Коханина М.И.
Рефлекторные влияния с рецепторов некоторых внутренних органов на лимфоток. –
«Труды института физиологии АН КазССР» ,1965, т.6, с.101-267
12. Беремжанова И.А. Экстеро
– и интероцептивные влияния на лимфоток в онтогенезе. – Труды института физиологии АН КазССР, 1965. Т.6,
с.268-424
13. Булекбаева Л.Э.
Сравнительная физиология лимфатической
системы. Изд-во «Наука» КазССР.
Алматы,1985
14. Мырзаханов Н.М. //Сб.
междунар. конф. «Проблемы лимфологии». –Новосибирск, 1987. –С.78.
15. Мырзаханов Н.М. Роль
лимфатических узлов и сосудов в
продвижении лимфы. –Вестн.НАН РК. – 1994(б). - №3. –С.70-76.
16. Хантурин М.Р. Эволюция
транспортной функции лимфатической
системы: Автореф., дисс. док. биол. наук. -Алматы,1996. –С.32
17. Газизова А.И., Алиев
А.Ф., Бекенова А.С. Архитектоника лимфатического русла некоторых органов
млекопитающих // Тезисы международного симпозиума «Физиология и патология
лимфатической системы». – 1997г Алматы. – С. 14.
18. Мырзаханов Н.М. //Сб.
междунар. конф. «Проблемы лимфологии». –Новосибирск, 1987. –С.78.
19. Бородин Ю.И., Пупышев
Л.В., Трсучев П.М. Эксперментальное исследование лимфатического русла.
Новосибирск, 1975. 138с.
20. Florey
H. Reactions of and absorptions by, lymphatics, wits special reference to those
of the diaphragm. “Brit. Y. Exper. Path.”, 1927, v.8, p.479.
21. Потапов И.А. Очерки
физиологии лимфообращения.
Алма-Ата,1977
22. Гареев Р.А., Лучинин
Ю.С., Ким Т.Д. Факторы лимфотока. Алма-Ата, 1982. 126с
23. Орлов Р.С., Борисова Р.П.,
Мандрыко Е.С. Сократительная и электрическая активность гладких мышц
магистральных лимфатических сосудов. – Физиол. журн. СССР, 1975, т.61, №7,
с.1045-1049
24. Лучинин Ю.С. К анализу
спонтанной активности лимфатических сосудов собак. – тр. ин-та физиол. АН
Каз.ССР, 1979, т.24, с. 38-49