*99599*
Doctor
of chemistry Skvortsov V.G.*, candidate of chemistry Ershov Ì.À.**,
Leontyeva À.Yu.*,
Mikhailova T.N.*, Novikov K.V.*
*Chuvash state teachers training university after named I.Y. Yakovlev
(CSTTU af. n. I.Y. Yakovlev), Cheboksary, the Russian Federation
** Chuvash state agricultural academy (CSAA),
Cheboksary, the Russian Federation
SYNTHESIS, STRUCTURE AND BIOLOGICAL ACTIVITY OF
BISETHYLENDIAMINECOPPER (II) NITRATE
It's
known, that copper as it’s a microelement and the organic derivates of ammonia
provide beneficial influence onto the growth and development of agricultural
crops. Their efficiency considerably increases if we use them not separately but as the parts of complexes. That’s
why the production of complex compounds based on the microelements and
physiologically active substances is an actual problem and it has scientific
and practical interest as the way of synthesis of new biogenic preparations and
their assortment extension.
Because
of it we’ve researched the properties of triple aqua system Cu(NO3)2–C2H8N2–H2O
by the methods of physicochemical analysis: isothermal solubility, densimetry, viscosimetry, refractometry and ðÍ-metry at 25˚C [1].
We’ve
taken preliminarily purified Cu(NO3)2·3H2O
of “p.f.a.”-mark (pure for analysis), ethylendiamine (EDA) of “c.p.”-mark
(chemical pure) for our experiments.
The
isothermal environment has been created in a water thermostat 1TJ–0–03 with the
accuracy ±0,1˚Ñ. The equilibrium in the system was established after 10-12 hours with
vigorous agitating and stirring. The separation of liquid phase from solid has
been done on the glass Schott
filter ¹ 4. The density has been measured by volume displacement meter (pycnometer), kinematic
viscosity – by viscosimeter VPJ-2, refractive index – by refractometer RF-454 BM, ðÍ – by universal ionomer
7Â-74.
The
iodometric analysis has shown us
the maintenance of copper
(II) ion, nitrogen of ethylendiamine has been determinated by potentiometry and collaterally by Kjeldahl
method. The structure of crystallizing solid phases has been
established by Schreinemakers’ method.
First,
there was a formation of blue sediment of copper (II) trihydroxonitrate Cu2(OH)3NO3
(gerhardtite). The increasing of ethylendiamine maintenance
in the reaction mixture leads
to the disolution of solid phase. All the copper
transfers to the solution of bisethylendiaminecopper (II) nitrate (BEDACN) as
the following complex Cu(NO3)2·2C2H4(NH2)2.
This transfer is accompanied by change of the color of solution from blue to
intensive violet.
The
synthesis of bisethylendiaminecopper (II) nitrate has been done by the
following way. The 81,6% water solution
of ethylendiamine (azeotropic mixture) has been added by drops from pipette to
diluted with water copper (II) nitrate in mole fraction nitrate:amine 1:1; 1:2;
1:4. The blue solution at this time has changed its color from blue to
intensive violet. It indirectly points onto the complex formation
process. The solution slowly vaporized in 8-10 days at room temperature with
dropping of violet prism crystals. Their chemical structure is:
It’s found, mass %: Cu – 20,21; N – 9,40.
For
Cu(NO3)2·2C2H4(NH2)2
it’s calculated, mass %: Cu – 20,65; N – 9,10.
The
individuality of complex has been confirmed by methods of chemical, X-ray
analysis and by crystal optics.
There
are the following characteristics of chemistry of our crystals [2] of
bisetylendiaminecopper (II) nitrate in our work: monoclinic syngony; Fedorov’s
group of symmetry Ð21/ñ; parameters of a crystal lattice a=8,302(10), b=10,052(4), ñ=8,065(10) Å, β=111°6´±12´,
formular unit of an elementary cell Z=2; calculation density ρcalc.=1,630 g/cm3, measurement
density ρmeas.=1,622 g/cm3.
The
four atoms of nitrogen from two molecules of EDA are coordinationally bonded
with central atom of copper in complex also nitrogen and copper atoms lie in
one plane. Length of bond between atoms of copper and nitrogen fluctuates from
2,01 to 2,04 Å. Bonds Cu–O directed perpendicularly to this plane and to
the side of two nitrate-groups; the length of this bonds is 2,59Å. In
general, two oxygen atoms and four atoms of nitrogen surrounding the complexing
ion form the deformed octahedron.
The
diffractograms of erased in a
powder crystals of our substance have been typed. X-ray phase analysis
has been made on diffractometer DRON-3
with the use of monochromatic CuKα-radiation (graphite
monochromator, U=35 kV, ian.=26
mA) Using noticed before parameters of lattice we’ve displayed the
diffractogram. Displaying has allowed us to specify the parameters of lattice
for researched crystals: a=8,326; b=10,035; ñ=8,044 Å (accuracy
±0,004Å) and β=111,04° (accuracy ±0,4°). This parameters have been calculated by their
reflection angle q>25°.
Using
the immersion method (liquid IJ-1) on the polarizing microscope MIN-8 we have
identified the indexes of refraction of crystals (they are prismatic and their
colour is violet). Their values are: ng=1,555±0,002 and nð=1,541±0,001.
The IR-spectrum changing of stretching vibrations ν(N-H)
and inward bending vibration δ(N-H) of EDA molecule shows us
the n-doublets of amino groups take an active part in complex formation.
IR-spectrums have been indicated on spectrophotometers SPECORD-75IR and UR-20
in vaseline oil and in the potassium bromide tablets.
Nitrate-ions NO3– are coordinated to
Cu2+ monodentativly by the Cu–O1 bond which is perpendicular
to the plane of chelate
ring (Fig.1).
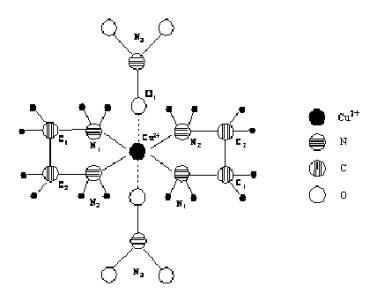
Fig.1 The
coordination scheme of NO3– ions and C2H4(NH2)2
molecules around the Cu2+ ion
Lengths of complex formative ion bonds with N and O atoms are:
Cu–N1=2,044Å
Cu–N2=2,012Å
Cu–Î1=2,593Å
Central copper atom’s coordination number is 6.
Physiological
tests with bisethylendiaminecopper (II) nitrate Cu(NO3)2·2C2H4(NH2)2
have been also taken place. We’ve made a wetting
of spring wheat seeds (grade
“Moskovskaya-35”), barley of an “Elf” grade, oats of an “Adamo” grade in 0,001%
water solution of in a lab and in a field. It’s established that the use of
BEDACN-complex increases the germinative energy of seeds on 9–22%, germinating
ability – on 14–23% (ð<0,05)* and it depends on a type of crops. The field experiments have shown the
use BEDACN-complex provides icreasing of germinative energy, germinating
ability of seeds, chlorophyll maintenance in leaves, ripening
acceleration and agricultural crops’ productivity
[3].
References
1. Pylchikova Yu.Yu., Diachkova I.M., Fedorova A.V. and oth. // Bulletin of CSTTU af. n. I.Y. Yakovlev – 2005. – ¹3. – P. 56–58.
2. Komiyama Y., Lingafelter E.C.
// The crystal structure of bisetylendiaminecopper (II) nitrate
// Acta Crystallogr. – 1964. – V. 17–19. – Ð. 1145.
3. Skvortsov V.G., Ershov Ì.À., Pylchikova Yu.Yu. and
oth. Means for preseeding processing of seeds of agricultural crops. – Application ¹2009140526/04(057622). – Decision
from 12.01.2011.