Acoustic studies
of the decomposition of hydrogen peroxide process
Dmitry M. Kuznetsov, Piotr N. Kozachenko, Irina A. Luganskaya, Victoria V. Aliluykina
Federal State Budgetary Educational
Institution of Higher Vocational Training "Novocherkassk State Land
Reclamation Academy"
Don State Technical University, Rostow on the Don, Russia, 344023, ul.
Strana Sowietow 1
Possibility of using acoustic emission (AE) method for studying passing in the liquid environment
with a gas phase allocation processes is shown by the example of decomposition
of hydrogen peroxide process research.
In the case of the heterogeneous
process of acoustic waves the nature of its generation is described partially
in works [1-5]. The given description can't be considered as being full as, the
generation of acoustic emission signals should be added to the described
mechanisms of acoustic emission [1,3,5]. Despite the mentioned complexities,
the method of AE should be accepted to be
interesting due to its decomposition of hydrogen peroxide. The research process is highly informative and
universal, allowing the registration of the processes which are passing in the decomposition
of hydrogen peroxide.
In connection with the above, the
purpose of the present work is to attempt the metrological evaluation of the
acoustic emission method’s applicability for the process of research decomposition
of hydrogen peroxide in conditions of variable
quantity of the catalytic amount. Manganese dioxide catalyses the decomposition
of hydrogen peroxide to oxygen and water [6]:
2 H2O2 → 2 H2O
+ O2
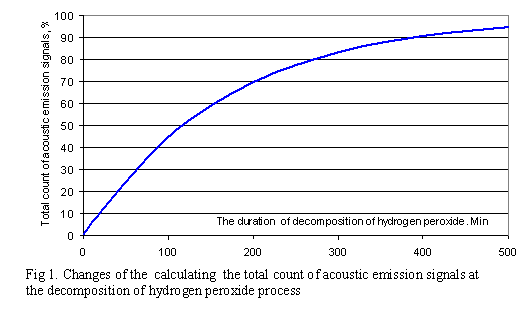
We will examine the change of evolution of the total acoustic emission signals’ and calculate it at the same time. The acoustic emission activity (for derivative of the total count by time) has an unstable character in the initial stage of the decomposition of hydrogen peroxide process.
This is a result of the simultaneous
superposition of the afore-mentioned phenomena: occluded gas release and gas cavitation bubbles process.
Approximately 1 minute after the beginning of the decomposition of hydrogen peroxide process, the activity is basically
defined by one factor – the reduction of the amount hydrogen peroxide (fig.1). The activity of
acoustic emission signals dN/dt naturally decreases and it’s proportional to
the speed of gas release :
dN/dt =
Kv, (1)
where Ê –proportionality ratio the speed of
the decomposition of hydrogen peroxide; v –
speed of the decomposition of hydrogen peroxide, g/s.
The activity dN/dt acoustic emission signals, is
naturally proportional to the speed of weight change:
dN/dt = K
m/t. (2)
Dividing variables and integrating
the equation (2), we receive the equation for the total count of acoustic
emission signals (fig.1):
N=Km Ln t – C (3)
A
schematic diagram of AE measurement system
used in the present work is shown in Fig. 2
In agreement with the given data we can confirm that: in spite of the fact that all registered parameters of the acoustic emission objectively reflect the dissolution process and the value of data spread doesn't make it a possible form to use such acoustic emission parameters such as ‘Duration’, ‘
Rise time’ and ‘the AE oscillations
rate’ for the
quantitative evolution of the dissolved substance’s weight.
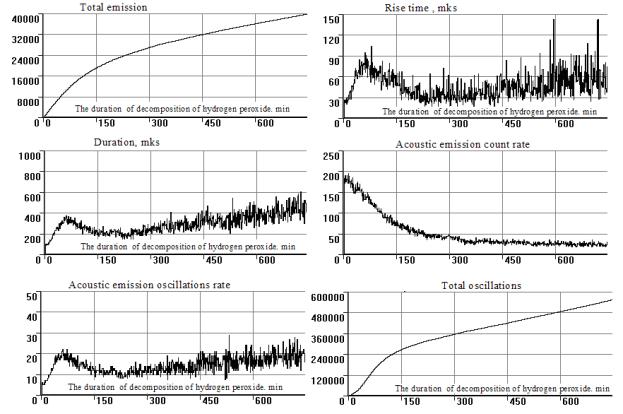
Fig 2. Changes in the
acoustic emission signals at the decomposition of hydrogen peroxide process
The most
reliable acoustic emission parameter, which can be used for a quantitative
evaluation of the dissolved substances is weight ‘the Total account’ of signals
Conclusion. The possibility
of using the acoustic emission method for study of the processes, passing in
fluid ambience with separation of the gas phase, was considered . In
particular, it was shown that in the process of removing gases out from water while
decomposition of hydrogen peroxide process several different
acousticses-emission pictures are identified. It evidences complexity and
multistage character of decomposition
of hydrogen peroxide process are. The data obtained
allow to forecast the sphere of using the AE method not only for study of the decomposition of hydrogen peroxide process,
but also for development of reliable and nondestroying method of controlling
the depth and fullness of chemical reactions and physical-chemical processes in
liquids.
References
1.
Kuznetsov D.M., Gaponov V.L. , Smirnov A.N. On the
possibility of studying the kinetics of chemical reactions in liquid medium
using the method of acoustic emission. "Engineering Physics" / / 2008
- ¹ 1-C16-21.
2.
Kuznetsov
D.M. Study of kinetics of crystal growth method of acoustic emissii.10
International Interdisciplinary Symposium "The order, disorder, and
properties of oxides» (ODPO-10) / / Proceedings of the Symposium Part2 .-
Rostov-on-Don - Loo, 2007, with .97-99.
3. Builo S.I., Kuznetsov D.M., Gaponov V.L., Trepachev V.V. Acoustic-Emission testing
and Diagnostics of the Dissolution Kinetics of Crystalline Components// Russian Journal of Nondestructive Testing 2012,
Vol. 48, No. 10, pp. 594-597.
4.
D. M. Kuznetsov ,
A. N. Smirnov , A. V. Syroeshkin Acoustic emission on phase
transformations in aqueous medium «Russian Journal of General Chemistry // 2008
Vol. 78 N11 p.2273-2281
5. S.I. Builo and D.M. Kuznetsov «Acoustic_Emission Testing and Diagnostics of the Kinetics of Physicochemical Processes in Liquid Media».- Russian Journal of Nondestructive Testing, 2010, Vol. 46, No. 9, pp. 686–691.
6. Martins R.R.L., Neves M.G.P.M.S., Silvestre A.J.D., Simoes M.M.Q., Silva A.M.S., Tome A.C., Cavaleiro J.A.S., Tagliatesta P., Crestini C. Oxidation of unsaturated monoterpens with hydrogen peroxide catalysed by manganese porphyrin complexes // Journal of Molecular Catalysis A: Chemical. 2001. Ò. 172. ¹ 1-2. Ñ. 33-42