Хімія
та хімічні технології/ 6. Органічна хімія
О. I. Panasenko, V. P.
Buryak, A. S. Gotsulya, S. M. Kulіsh
Zaporozhye State Medical University
Basic optical characteristics of
the electronic absorption spectrum (BOCEASP) of drugs. Determination BOCEASP of
drugs, containing in the molecule non-condensed furan cycle
Development
of pharmaceutical chemistry put on the next tasks in front of the molecular
spectrophotometry: calculating of the electronic condition, prediction and
explanation of different properties of complex polymolecular organic compounds.
To solve these problems, the most commonly used method of ultraviolet
spectrophotometry, which on the basis of the BOCEASP calculation can explain
the electronic structure of studied compound and to determine the molecule
fragment that determines the pharmacological activity of the molecule.
Keywords: pharmaceutical chemistry, molecular
spectrophotometry, optical characteristics, UV spectra.
BOCEASP included in the
State Pharmacopoeia of Ukraine [6] are the wavelengths λmax (in
nm) and specific absorption index -  . At the same time for chemical reagents, dyes and other chemical
compounds in the last 10 years have been widely used also other BOCEASP: wave
number at the maximum of absorption - νmax (cm-1);
bold absorption band Δv1/2 (cm-1); the integral
intensity of the absorption band (in L-mol•cm2), the oscillator
strength of the electronic transition - f; the transition matrix element - Mik
[4, 5, 8, 9].
. At the same time for chemical reagents, dyes and other chemical
compounds in the last 10 years have been widely used also other BOCEASP: wave
number at the maximum of absorption - νmax (cm-1);
bold absorption band Δv1/2 (cm-1); the integral
intensity of the absorption band (in L-mol•cm2), the oscillator
strength of the electronic transition - f; the transition matrix element - Mik
[4, 5, 8, 9].
Introduction of the
specified BOCEASP in a practice of pharmaceutical analysis is undoubtedly
expedient, as these quantities may be used for drugs identification, similar in
their chemical structure; for deep study of the molecules electronic structure,
and also may be used as important parameters for communication establishment
between the structure and pharmacological activity.
The purpose of work. In our work cycle we
set the aim to study the OOHESP of twenty-nine drugs, containing in their
molecule -O and -O, N-heterocycles, and also for several hormonal drugs
comparison, characterized by very close in value quantities λmax.
Materials and methods. All researched drugs,
containing in a molecule non-condensed furan cycle, completely comply with the
requirements of the State Pharmacopoeia, and their standard samples were
obtained from the State Enterprise Scientific-Expert pharmacopoeia center of
Ukraine. In all cases, as a solvent we used 95% ethyl alcohol.
UV-spectra of test
compounds were measured by means of the spectrophotometer SPECORD 200-222U214
(Germany).
Results and their discussion.
We have defined and calculated BOCEASP of UV-spectra of twelve drugs in
ethanol, which are furan derivatives (furagine, furagine soluble, furazoline,
furazolidonee, furadonine, furatsiline, nitrofurilene, furazonole, fubromegane,
furasemide, larusane, ftorafure) and of two model compounds (uracile,
5-fluorouracil).
The absorption bands of
all researched drugs with non-condensed furan cycle are interferential or
high-intensity, as indicated by the molar absorption coefficient, the value of
which ranges from 4500 to 34000 (the third and the first absorption bands of
furosemide). It should be noted that for studying of 5-nitrofuran derivatives
these quantities Emax are situated in more narrow ranges, namely
from 8300 (first nitrofurilene band) to 25500 (the third furagine band). This
indicates that all absorption bands may not correspond to π→π*
transitions, which are characterized by low-intensive bands [7].
The half-width of the
absorption band is broad (from 2970 to 9850 cm-1) for twenty from
the twenty-seven observed bands. Very broad, i. e. with Δν1/2
= 10,000 cm-1 and higher, is the first furagine band and its
potassium salt, furazolidone, furadonine, nitrofuragine, and also first and
second furazolidone bands (Table. 1).
Drugs, which are
5-nitrofurane derivatives, present relatively homogeneous group by quantities
of the integral intensity of the A absorption bands. All these quantities are
high and situated in the ranges from 0,87 ∙ 108 to 1,49 ![]() 108, that indicates
on a high probability of electrons transition, causing the appearance of
absorption bands of nitrofurane derivatives. Exclusion is the second absorption
band of furadonine, characterized by the average quantity of the integral
intensity (A = 0,67 ∙ 108).
108, that indicates
on a high probability of electrons transition, causing the appearance of
absorption bands of nitrofurane derivatives. Exclusion is the second absorption
band of furadonine, characterized by the average quantity of the integral
intensity (A = 0,67 ∙ 108).
It should be noted,
however, that for the absorption band of 5-nitrofurane derivatives with λmax
in area 332-385 nm, quantities A are the highest. Namely this band arises as a
result of coupling reaction in chromophores, containing in their structure the
nitro group. Presence of these chromophores causes the antimicrobial activity
of 5-nitrofurane derivatives that is why we can consider them as pharmacophores
(or their part).
Drugs, containing in
their molecules a furan cycle, but without a nitro group (fubromegane,
furosemide, larusane, ftorafure) containing in UV-spectra four bands with high
quantities of the integral intensity (from 0,89 ∙ 108 to 1,88
∙ 108) and at the band with the average A quantities (from
0,22 ∙ 108 to 0,63 ∙ 108). Thus, the
transitions of electrons, causing the appearance of these bands are probable or
highly probable.
The lowest quantity A,
namely 0,22 ∙ 108 is observed for the middle band absorption
band of furosemide with λmax at 334 nm. Maximum relates to 1Lb
band, which cannot has any direct connection with the pharmacophore, which
causes the diuretic effect of the drug.
Quantities of the
oscillator force f for all absorption bands of 12 studied drugs with
non-condensed furan cycle are situated in the ranges from 0,24 to 2,00 (Table.
1). Decimals logarithms of these quantities ![]() are from 0,62 to 0,30 at quantities ln εmax, located in the ranges from 3,65 to 4,54. Thus, in accordance with the
scale of the oscillator strengths of molecular electronic transitions, composed
by Kashe Rawls [10], electron transitions, causing the appearance of the
absorption bands of 5-nitrofurane, fubromegane, furosemide, and lorisane and
ftorofure derivatives are permitted.
are from 0,62 to 0,30 at quantities ln εmax, located in the ranges from 3,65 to 4,54. Thus, in accordance with the
scale of the oscillator strengths of molecular electronic transitions, composed
by Kashe Rawls [10], electron transitions, causing the appearance of the
absorption bands of 5-nitrofurane, fubromegane, furosemide, and lorisane and
ftorofure derivatives are permitted.
Found
quantities of Mik matrix element transition are high and compose from 3,6
∙ 10-18 (second absorption band of the furosemide) to 6,46
∙ 10-18 (absorption band of the larusane). These quantities
are not directly proportional to the reactionary ability, corresponding to the
chromophore or pharmacophore, but in certain degree, indicate that these groups
of atoms are highly reactive.
Table 1
Optical characteristics of the electronic absorption
spectra of drugs, containing in their molecule non-condensed furan cycle
|
№ o\o |
Drugs |
Solution conc. |
λ, nm. |
��, сm-1 |
|
|
|
А |
f |
Mik |
|
1. |
Furagine |
1 mg % ethanole |
245 292 380 |
40820 34250 26320 |
0,86 2,11 2,55 |
3,94 4,33 4,41 |
10720 6060 5460 |
0,99 1,37 1,49 |
1,07 1,46 1,59 |
4,30 5,50 6,54 |
|
2. |
Dissolved furagine |
1 mg % ethanole |
232 292 385 |
43100 34250 25970 |
1,03 1,92 2,39 |
4,01 4,28 4,38 |
12120 5980 5770 |
1,34 1,23 1,48 |
1,43 1,31 1,58 |
4,85 5.21 6,56 |
|
3. |
Furazole |
1 mg % ethanole |
234 265 356 |
42740 37740 28090 |
0,88 0,95 1,57 |
3,94 3,98 4,20 |
13460 10150 6180 |
1,27 0,98 1,04 |
1,35 1,05 1,11 |
4,70 4,44 5,34 |
|
4. |
Furazolidone |
1 mg % ethanole |
234 265 356 |
42740 37740 28090 |
0,88 0,95 1,57 |
3,94 3,98 4,20 |
13460 10150 6180 |
1,27 0,98 1,04 |
1,35 1,05 1,11 |
4,70 4,44 5,34 |
|
5. |
Furadonine |
1 mg % ethanole |
225 272 356 |
44440 36760 28090 |
0,85 0,85 1,30 |
3,96 3,96 4,15 |
10000 7380 6370 |
0,91 0,67 0,87 |
0,97 0,72 0,93 |
3,92 3,70 4,62 |
|
6. |
Furaciline |
1 mg % ethanol |
263 364 |
38020 27470 |
1,17 1,82 |
4,07 4,26 |
9850 5360 |
1,13 1,05 |
1,21 1,13 |
4,74 5,40 |
|
7. |
Nitrofurilene |
1 mg % ethanol |
244 347 |
40980 28820 |
0,83 1,97 |
3,92 4,30 |
10000 5270 |
0,89 1,11 |
0,95 1,19 |
4,05 5,41 |
|
8. |
Furazonale |
1 mg % ethanole |
239 332 |
41840 30120 |
1,12 1,99 |
4,05 4,30 |
7620 5430 |
0,91 1,16 |
0,98 1,23 |
4,10 5,39 |
|
9. |
Fubromegane |
1 mg % ethanole |
220 265 |
45450 37740 |
1,68 1,72 |
4,23 4,24 |
5680 4810 |
1,02 0,89 |
1,10 0,95 |
4,10 4,22 |
|
10. |
Furocemide |
1 mg % ethanole |
230 274 334 |
43880 36500 29940 |
3,44 1,99 0,45 |
4,54 4,30 3,65 |
5110 2970 4540 |
1,88 0,63 0,22 |
2,00 0,67 0,24 |
5,71 3,61 2,39 |
|
11. |
Larusane |
1 mg % ethanole |
334 |
29940 |
2,96 |
4,47 |
5190 |
1,64 |
1,76 |
6,46 |
|
12. |
Furacile |
1 mg % ethanole |
258 |
38750 |
0,79 |
3,90 |
4870 |
0,42 |
0,48 |
3,00 |
|
13. |
5-Furfurole |
1 mg % ethanole |
267 |
37450 |
0,69 |
3,84 |
5060 |
0,37 |
0,40 |
2,70 |
|
14. |
Ftorafure |
1 mg % ethanole |
270 |
37050 |
0,85 |
3,43 |
4710 |
0,43 |
0,45 |
3,00 |
For a more
completely interpretation of ftorofurane UV-spectra were determined uracil and
5-fluorouracil BOCEASP in ethanol solutions for a comparison of these compounds
as models. In addition, we calculated these characteristics also for
cyclohexane solution of ftorfurane.
During the
transition from non-polar solvent (cyclohexane) to polar (ethanol) absorption
maximum, and also the integral intensity of the A absorption band, the
oscillator strength of the electronic transition f and the transition matrix
element Mik are almost unchanged within the limits of experimental errors. This
proves that the band at 270 nm corresponds to electrons transfer of the
chromophore, not π→π* local excitation of the nucleus.
Similar quantities A, f and Mik for uracil (1), 5-fluorouracil (2) and
ftorfurane (3) are evidence that all of these compounds are characterized by a
one chromophore, namely:
![]()
Introduction of the
electron-donor F atom at C5 promotes to the electrons transfer,
resulting in a bathochromic displacement of maximum on + 9 nm or ≈ 3,7
kkal/mol. Simultaneously, a fluorine atom violates the complementary of a
molecule, as a result is observed a decrease of the extraction in 1.4 times.
The band at ≈270 nm cannot correspond to the chromophore:
![]()
This chromophore is
contained in the tautomeric form of ftorafurane (4), because in this case the
fluorine atom would not affect the position of the absorption maximum.
Further substitution of
H atom at the N1 atom in a molecule (2) on the tetrahydrofurane
residue, only in a small degree promotes to the transfer of electrons in the
chromophore.
High quantities of the
oscillator strength (0,40-0,48), expressed as the ratio of the average value of
the oscillating charge in the molecule to the charge of a single electron,
indicates not only on the allowed transition, also on high probability of
electron transfer in the chromophore. Thus, uracil in ethanole solution exists
in a form 1,2,3,4-tetragidropirimidindiol-2,4, instead of 2,4-dioksipirimidine.
Based
on the identity of spectrums (4) in etalon and aqueous solutions we can suggest
that (4) is not exposed dissociation to the ions in the water, in alkaline
solutions is observed at 220-240 nm a very strong increase of the extraction,
indicating on a salt formation of type (5). As is well known, the maximum,
corresponds to the electrons transfer in the amide chromophore -NC=O, is
situated below 200 nm [8]. This electrons transfer will be greatly increase at
the ions formation of (5) form with the negatively charged nitrogen atom. About
the formation of similar mono-anions informs R. A. Juyk and coauthors [1].
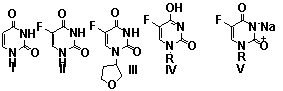
Pic.
1. Structure of the researched compounds
On
the basis of presented facts we can judge that the physiologically active
moiety contained in the molecules of fluorouracil and ftorafure is a
pharmacophore [3].
Conclusions
1. Established
that Δλ1/2 and the integral intensity of the
absorption bands A, the oscillator strength f and Mik matrix element transition
can serve as important constants of drugs for their identification and the
establishment of deeper connection between spectrums and the structure of the
molecule.
2. For drugs,
5-nitrofurane derivatives, the most characteristic is the third absorption band
with very high values A and f; for specified compounds chromophores and
pharmacophores apparently coincide.
3. All electrons
transitions, causing the appearance of the absorption bands of drugs,
containing in the molecule O-heterocycles are permitted and in most cases, have
a high probability.
4. On the basis
of OOHESP studying possible we can claim that the pharmacophore in a molecule
of 5-fluorouracil and ftorafure is a group:.
![]()
LITERATURE
1. Analogues of
pirimidine nucleozide. The kinetics of acid hydrolysis of N1-(2-furanidil)
and N1-(2-piranidil) derivatives of uracil, thymine and
5-fluorouracil / R. A. Zhuk, A. E. Berezina, T. T. Volynskaya, S. A. Tiller //
Chemistry of heterocyclic compounds. - 1990. - № 4. - P. 550-553.
2. Brand J. The
spectroscopy application in organic chemistry / J. Brand, G. Eglinton. - M. : Wor. - 1997. - 280 p.
3.
Buryak V. P. About the structure of antitumor agent - phtorafura / V. P.
Buryak, N. M. Turkevich // Scientific proceedings I. P. Pavlov Ryaz. Med. Inst.
- Ryazan. - 1994. - T.70. Questions about new drugs development. - P. 95-99.
4.
Buryak V. P. Application of the main characteristics of the electronic
absorption bands in pharmacy / V. P. Buryak // Pharmacy. - 2001. - V. 49, № 2.
- P. 19-23
5.
Vasiliev V. P. Theoretical foundations of physical and chemical methods of
analysis / V. P. Vasiliev - M .: Higher School. - 2005. - 184 p.
6.
State Pharmacopoeia of Ukraine / State enterprise "Scientific-expert
pharmacopeia center" 1-st public. - Kharkiv: RІREG. - 2001. - 556 p.
7.
Saidov G. V. Practical manual to molecular spectroscopy / G. V. Saidov - L .:
Leningrad State University. - 2000. - 136 p.
8.
Sverdlova O. V. Electronic spectra in organic chemistry / O. V. Sverdlova - L
.: Chemistry. - 1995. - 248 p.
9.
Physical and chemical methods of research in organic and biological chemistry /
T. Ya. Paperno, V. P. Pozdnyakov, A. A.
Smirnova, L. M. Yelagin. - M .: Education. - 2007. - 176 p.
10.
Kasha M. Correlation of orbital classification of molecular electronic
transitions with transition mechanism: the aromatic amines / M. Kasha, HR Rawls
// Photochemical and Photobiology. - 2008. - Vol. 52. - № 6. - P. 561-569.