Технические науки/1. Металлургия
Zhiguts Yu.Yu.1, Gabovda O.V.2, Antalovskyi V.V.1
1Uzhgorod National
University, Ukraine
2Mukathevo State University, Ukraine
THE SYNTHESIZED ALLOYS USING THE
GEOMETRIC THERMODYNAMIC METHODS
The problem of finding the real internal combustion temperatures for
exothermic mixtures is that the adiabatic combustion temperatures reach 2400–3500
К, while when being used in combined exothermic reactions with chromium oxide
it is 6300 К that exceeds the luminance temperature of the Sun surface. Therefore,
the geometric thermodynamic methods are the most expedient to find the alloy
phase composition. One has also to take into account that now they are
intensively developed and corrected.
The
geometric thermodynamic methods developed by the authors allow the alloy phase
composition to be clearly found dependent of the chemical composition of the
reaction components and of the temperature. The data obtained by the authors
are confirmed by the experimental studies on the phase composition [1].
Example of calculation. When using a
classical Hillert method [1] and its modifications [2] the most important
initial quantity in calculating activities ai
along the conoid of the two-phase region of a given isothermal section of the
three-component (angle h of the
system h-i-j) phase diagram is the
activity aiº along
the cognominal conoid of the binary system hi.
However, this assumes that the above two-phase region is located close to the
diagram hi. And what about the
opposite?
In this
case one has to know the activity aiº
along at least one conoid of the three-component two-phase region α+β (see fig. 1), and then
this conoid will be taken as the basic one though it is inclined towards the
axis hi (νj=0) and the latter is spaced from the region α+β.
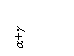
![]()
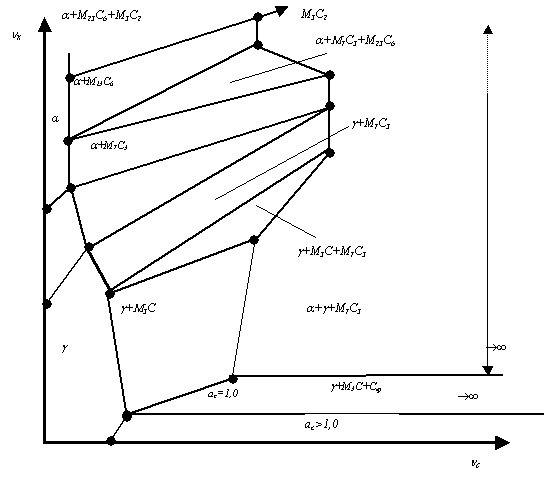

Figure 1. The isothermal
section scheme for the phase diagram of the alloys Fe-C-X, in which the
graphite at aC>1.0 is produced (provided the graphite is chosen
as the carbon standard state) and the carbide phases M3C
(cementite), M7C3, M23C6 and M3C2
are produced
The isothermal section in fig. 1 is plotted in
the Schreinemakers coordinates because this enables a very simple and exact
equation to be used in calculations, in which there is no node or figurative
alloy point coordinates [1]:
![]() , (1)
, (1)
where aiº is the component
activity along the basis ("zero") conoid at Xj=0 (the binary system hі, when the region α+β
is adjacent by this conoid to the binary diagram hi); ![]() is the tangent
of the angle of the slope of the two-phase equilibrium conoid in the system
under study hij.
is the tangent
of the angle of the slope of the two-phase equilibrium conoid in the system
under study hij.
All
this, in turn, allows one to calculate ai
along the conoid in the case shown in fig. 1 using the following equation:
![]() (2)
(2)
where ńі and ńо are the tangents of
the angles of slope of the conoids αΙβІ
and α0β0,
respectively; aiδ
is a known value ai along
the basis conoid α0β0.
Similarly,
for the conoid αІІβІІ,
the calculation is carried out according to equation:
![]() (3)
(3)
where ![]() is the αІІβІІ
conoid slope.
is the αІІβІІ
conoid slope.
It is
easy to show that the Hillert equation [1] that involves the node coordinates
(not saying about the vast approximations required for its derivation) is not
fitted for such calculations because at the transition from the conoid α0β0 to
the conoid αΙβΙ
and then to the conoid αΙΙβΙΙ
it is changed essentially.
Figure 1
shows a schematic example of the use of the developed method for the isothermal
section (in the supercritical temperature region) in the Fe-C-X-like system (where X
is manganese, chromium or their analogues). In the two-phase region g+Cгр austenite+graphite), the
carbon activity exceeds 1.0. In the conoid triangle g+M3C+Cгр (austenite+doped
cementite+graphite), the carbon activity is by definition aC = 1.0. Above this triangle, aC<1 and these alloys are
not capable of graphitizing. The conoid g+M3C of the above triangle is a
basic conoid α0β0
(fig. 1) along it aC = 1.0.
The use of calculations using equations (2) and (3) in the region g+M3C (just here the activity
decreases monotonously down to aC < 1.0)
up to the conoid g+M3C of the next conoid
triangle g+M3C+M7C3 (the activity aC decreases because ń, and, respectively ń-ń0, increase
progressively). In this triangle, the carbon activity is constant and this
allows us to use equations (1) and (2) in the calculations for the triangle g+M7C3, where activity aC keeps decreasing with
increasing alloy doping degree. The triangle α+γ+M7C3, in the inner part of
which the carbon activity is constant, allows one to transfer the value aC from the right bottom
conoid of triangle to the upper conoid and to continue calculations using
equations (2) and (3) in the triangle α+M7C3.
Continuing the above construction for the regions lying above the triangle α+M23C6+M7C3,
the tetragon α+M23C6
and the triangle α+M23C6+M3C2,
one may extend these calculations to the highly-chrome alloys, though with very
high degree of approximation, because the Hillert method is, in principle,
elaborated perfectly for the dissolved systems only.
Analysis of the theoretical results. Thus, the
successful application of the approximations introduced into the Hillert
equation has a certain theoretical basis. The use of the Schreinemakers
coordinates instead of the conditional ones provides a unique possibility to
simplify and solve the Hillert equation and reduces the number of
approximations to the two only, both of them being completely confirmed in the
most cases of practical application. The
relationships successfully found may give a basis for the certain types of
calculations of the ternary phase diagrams provided the principal double
diagram and some thermodynamic data related to the alloy element distribution
over the phases are known. The results of these calculations are used in the
high-temperature studies of alloys and in the cast alloy synthesis by using the
combined processes.
The
original Hillert method is not suitable to solve a series of tasks related to
the high-temperature alloy synthesis, unlike the modified method, that uses the
orthogonal Schreinemakers coordinate frame. The reason is that the modified
method does not require determination and use of the relevant node coordinates
in calculations.
Література:
1. Жигуц,
Ю. Ю. Технології отримання та особливості
сплавів синтезованих комбінованими процесами [Текст] / Ю. Ю. Жигуц, В.Ф. Лазар. Ужгород: Видавництво «Інвазор»,
2014. – 388 с.
2. Жигуц, Ю. Ю. Методика розрахунку складу екзотермічних
шихт на основі термохімічного аналізу [Текст] / Ю. Жигуц, В. Широков //
Машинознавство. – 2005. – № 4. – С. 48-50.