Cand. Sci. (bio) Petrusha Yu.Yu.
Zaporizhzhya National University, Ukraine
RESEARCH
OF TOXIC ACTION OF S-PYRIDINE-MERCAPTOACIDS DERIVATIVES
INTRODUCTION.
Pyridine chemistry for the last decades grew into one of the widest
division of chemistry of heterocyclic substances. The
heterocyclic system of pyridine is basis of many medicinal facilities that have
a wide spectrum of pharmacological action. Substances, that show an
antioxidant, antimicrobial, fungicide and other types of activity, were found
among them. The possibility of using pyridinethyoles to receive the
preparations with a cardioactive, analgetic, neurotropic action is
especially valuable. Well-known facilities are on the basis of nicotinic and
isonicotinic acids.
Clearly the practical value of substances of the
indicated row does not finish on that examples and is not exposed yet. That’s
why the pyridine system nowadays pretends on an intent attention of
researchers. So the purposeful organic synthesis is the most perspective way to
develop the pyridine chemistry. The research of biologically active substances in the rows of insufficiently known
S-pyridinederivates is important and has the
theoretical and practical value [1, 2].
The introduction of biologically active
substances, including regulators (stimulators) of height and development of
plants (RPG) is perspective in a nowadays conditions. Using these preparations
helps us to realize better the genetic possibilities, to rise the plant
firmness against the stress factors of biotic and abiotic nature and at last to
increase the harvest and to improve its quality [3]. Synthetic RPG from the
derivative nitrous heterocyclic group, such as pyridine, are perspective to use
in agriculture [4].
Results of
researches of the last years [5, 6] show that combination of nitrogen-containing heterocycle and mercaptocarbonic acids influence on strengthening of biological
action or appearance of new effects. Therefore a search of new bioactive
substances containing in the molecule a heterocycle and a deputy with high
antioxidant properties such as succinic acid or aminoacid L-cysteine is
perspective.
That’s why on the
basis of those studies [7] the di-Na-salt of 2-(pyridine-4-iltio)succinic acid
and dihydrochloride S-(pyridine-4-il)-L-cysteine were selected for the deep research.
RESEARCH
MATERIALS AND METHODS. Structure of the
synthesized substances is confirmed by data of PMR-, ІR-spectroscopy and element analysis, and
the cleanness – by the method of thin-layer chromatography (TLC).
The sharp toxicity was studied on white intact adults
bisexual mouse weighing 20±3,0 g by means of
Prozorovsky V. B. tabular express-method to determinate middle effective measures of
influence on biological objects [8.
Researches of
antibacterial activity of substance were conducted in vitro on methodology of the serial double breeding in a liquid
nourishing environment (broth of Hоttingеr) [9]. Experiments executed on the 4 strain of bacteria, from that 2
cultures were gram-positive (Bacillus subtilis, Staphylococcus aureus),
and other 2 – gram-negative (Escherichia coli, Pseudomonas aeruginosa).
The estimation of results was carried out depending on intensity by oppressions
of height of that or other culture of bacteria by a substance with a maximal
concentration 500 mcg/ml.
The investigation
of synthesized substance influence on a fission and cell growth (cytotoxicity) was done
on a root test on the sprouts of Cucumis
sp. (during experience used the cucumbers of sort "Competitor")
[10]. Cytotoxicity of substance was estimated
after reduction of the marked parameters in an experiment comparatively with
control. Control (water)
indexes are taken for a zero.
RESULTS
AND DISCUSSION. The studies of sharp toxicity proved that di-Na-salt of
2-(pyridine-4-iltio)succinic acid is more or less safe according to the
classification of Sidorov at intra-abdominal introduction to the wide range of
doses. Animals were put the dose 4000 mg/kg and they were alive and active
through 12, 24 hours and on a 14 day. Reflex
activity, breathing depth and frequency of mice was not damaged. The reception
of water, meal, egestion were not changed. Weight was not changed in comparison
with the control group of animals. LD50 of di-Na-salt of
2-(pyridine-4-iltio)succinic acid presents
4960±66 mg/kg.
The studies of
sharp toxicity proved that dihydrochloride S-(pyridine-4-il)-L-cysteine
is low-toxic according to the classification of Sidorov at intra-abdominal
introduction. LD50 of dihydrochloride
S-(pyridine-4-il)-L-cysteine presents 832±81 mg/kg.
Research of
antibacterial and cytotoxic action of di-Na-salt
of 2-(pyridine-4-iltio)succinic acid confirmed
the absence of toxic effects. It is found out that investigated substance does
not have antibacterial activity at concentrations 500 mcg/ml. Cytotoxic action of di-Na-salt of
2-(pyridine-4-iltio)succinic acid was not marked, but even for concentrations
500 mcg/ml this substance stimulates fission and cell growth of sprouts of Cucumis sativus (figure 1).
% hypocotyl
length length of the
main root length of a
zone of lateral roots quantity of
lateral roots Concentration, mkg/ml
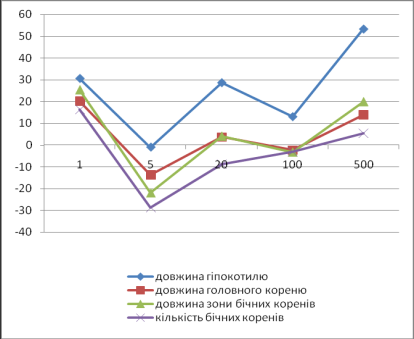
Figure 1. Influence of di-Na-salt of 2-(pyridine-4-iltio)succinic acid
on a fission and cell
growth of sprouts of Cucumis sp.
Research of
antibacterial action of dihydrochloride S-(pyridine-4-il)-L-cysteine confirmed the absence of toxic effects. It is found out that
investigated substance does not have antibacterial activity at concentrations
500 mcg/ml.
The research of the efficiency of dihydrochloride
S-(pyridine-4-il)-L-cysteine (table 1) shows that the offered substance has a
considerable grow-stimulating activity. It has ability to increase the length
of main root and the amount of lateral roots of cucumber, that promotes the
quick growing of leaves and productivity and viability of plants.
Table 1
Indexes of height
stimulation of cucumbers under the influence of
dihydrochloride
S-(pyridine-4-il)-L-cysteine
|
Parameters |
Concentration,
mcg/ml |
Control (distilled water) |
||||
|
1 |
5 |
20 |
100 |
500 |
||
|
Length of main root, mm |
53,4±1,6* |
47,6±2,1 |
48,6±2,2 |
53,4±2,0* |
31,8±1,0 |
41,9±2,0 |
|
Length of height zone of lateral roots, mm |
21,5±1,2* |
17,8±0,9 |
17,1±1,1 |
20,7±1,0* |
15,0±0,6 |
14,9±0,8 |
|
Amount of lateral roots |
10,3±0,4 |
10,5±0,6 |
9,6±0,7 |
10,9±0,5 |
7,5±0,5 |
9,0±0,3 |
Primark: Р<0,05; * −
concerning control. Control (the distilled water) it is accepted for zero.
CONCLUSIONS
1. It was found out that
di-Na-salt of 2-(pyridine-4-iltio)succinic acid is relatively safe at
intra-abdominal introduction to the wide range of doses (her LD50 =
4960 mg/kg). Research of its antibacterial and cytotoxic
action confirmed absence of toxic effects.
2.
Di-Na-salt of
2-(pyridine-4-iltio)succinic acid is untoxic and can come forward as a
substance for further researches to create new effective pharmaceutical
preparations on its basis.
3.
Dihydrochloride
S-(pyridine-4-il)-L-cysteine is capable to increase the length of the main root and quantity of
lateral roots of sprouts of gourd family that promotes more faster development
of gypocotile and leaves and increases the productivity and viability of the
plants.
REFERENCES
1.
Петруша Ю. Ю. Біологічна активність S–похідних піридин-2(4)-ілтіолів (огляд літератури)
/ Ю.Ю. Петруша, Л.О. Омельянчик, О.А. Бражко // Вісник Запорізького
національного університету. – 2008. –
№ 2. – С. 156–162.
2.
Петруша Ю. Ю. Піридин-2(4)-іл-тіоли як основа для створення біологічно активних речовин
/ Ю.Ю. Петруша, Л.О. Омельянчик, О.А. Бражко // Вісник Донецького університету.
– 2009. – № 1. – С. 311–316.
3.
Калiнiн Л. Ф. Застосування регуляторiв росту в сiльському господарствi / Л.Ф. Калинин.
– К.: Урожай, 1989. – 168 с.
4.
Жминько О. П. Токсикологическая характеристика
регуляторов роста растений – производных N-оксид пиридина: дис. … канд. биол.
наук:
14.03.06. «Токсикология»
/ Олеся Петровна Жминько. – Киев, 2007. – 226 с.
5. Бражко О. А. Біологічно активні похідні хіноліну та акридину з азото- та
сірковмісними функціональними групами: автореф. дис. на здобуття наук. ступеня
док. біол. наук. – Запоріжжя, 2005. – 43 с.
6. Бєленічев І. Ф. Дослідження антиоксидантної дії
хіназоліл-4-(хінолін-4)-тіо-α(β)-карбонових кислот та їх похідних за
умов ініціювання вільно-радикальних процесів in vitro та моделюванні ішемії головного мозку / І.Ф. Бєленічев, С.І. Коваленко,
О.А. Бражко, О.В. Карпенко // Ліки. – 2001. – № 5–6. – С. 28–33.
7. Петруша Ю. Ю. Біологічна активність деяких S-гетерилзаміщених
L-цистеїну та їх аналогів / Ю.Ю. Петруша, Л.О. Омельянчик, О.А. Бражко, М.П.
Завгородній // Ukrainica Bioorganica Acta. – 2011. – № 2. – С. 46–52.
8. Прозоровский В. Б. Табличный
экспресс-метод определения средних эффективных мер воздействия на биологические
объекты / В.Б. Прозоровский // Токсикологический вестник. – 1998. – №1. – С.
28–32.
9. Биргер М. О.
Справочник по микробиологическим и вирусологическим методам исследования / О.
М. Биргер. – М.: Медицина, 1982. – 464 с.
10. Иванов В.
Б. Клеточные основы роста растений / В.Б. Иванов. – М.:
Наука, 1974. – 222 с.